Abstract
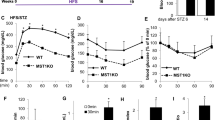
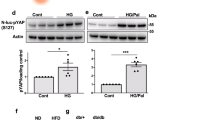
Apoptotic cell death is a hallmark of the loss of insulin-producing beta cells in all forms of diabetes mellitus. Current treatments fail to halt the decline in functional beta cell mass, and strategies to prevent beta cell apoptosis and dysfunction are urgently needed. Here, we identified mammalian sterile 20–like kinase-1 (MST1) as a critical regulator of apoptotic beta cell death and function. Under diabetogenic conditions, MST1 was strongly activated in beta cells in human and mouse islets and specifically induced the mitochondrial-dependent pathway of apoptosis through upregulation of the BCL-2 homology-3 (BH3)-only protein BIM. MST1 directly phosphorylated the beta cell transcription factor PDX1 at T11, resulting in the latter's ubiquitination and degradation and thus in impaired insulin secretion. MST1 deficiency completely restored normoglycemia, beta cell function and survival in vitro and in vivo. We show MST1 as a proapoptotic kinase and key mediator of apoptotic signaling and beta cell dysfunction and suggest that it may serve as target for the development of new therapies for diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kurrer, M.O., Pakala, S.V., Hanson, H.L. & Katz, J.D. Beta cell apoptosis in T cell–mediated autoimmune diabetes. Proc. Natl. Acad. Sci. USA 94, 213–218 (1997).
Mathis, D., Vence, L. & Benoist, C. Beta-cell death during progression to diabetes. Nature 414, 792–798 (2001).
Butler, A.E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 (2003).
Rhodes, C.J. Type 2 diabetes—a matter of beta-cell life and death? Science 307, 380–384 (2005).
Donath, M.Y., Storling, J., Maedler, K. & Mandrup-Poulsen, T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J. Mol. Med. 81, 455–470 (2003).
The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann. Intern. Med. 128, 517–523 (1998).
Lenzen, S., Drinkgern, J. & Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 20, 463–466 (1996).
Ling, P., Lu, T.J., Yuan, C.J. & Lai, M.D. Biosignaling of mammalian Ste20-related kinases. Cell. Signal. 20, 1237–1247 (2008).
Avruch, J. et al. Protein kinases of the Hippo pathway: regulation and substrates. Semin. Cell Dev. Biol. 23, 770–784 (2012).
Lee, K.K. et al. Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 16, 3029–3037 (1998).
Kakeya, H., Onose, R. & Osada, H. Caspase-mediated activation of a 36-kDa myelin basic protein kinase during anticancer drug-induced apoptosis. Cancer Res. 58, 4888–4894 (1998).
Bi, W. et al. c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at serine 82. J. Biol. Chem. 285, 6259–6264 (2010).
Cheung, W.L. et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113, 507–517 (2003).
Jonsson, J., Carlsson, L., Edlund, T. & Edlund, H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606–609 (1994).
Stoffers, D.A., Zinkin, N.T., Stanojevic, V., Clarke, W.L. & Habener, J.F. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat. Genet. 15, 106–110 (1997).
Johnson, J.D. et al. Increased islet apoptosis in Pdx1+/− mice. J. Clin. Invest. 111, 1147–1160 (2003).
Stoffers, D.A., Ferrer, J., Clarke, W.L. & Habener, J.F. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat. Genet. 17, 138–139 (1997).
Brissova, M. et al. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 277, 11225–11232 (2002).
Ardestani, A. et al. Neutralizing interleukin-1β (IL-1β) induces beta cell survival by maintaining PDX1 protein nuclear localization. J. Biol. Chem. 286, 17144–17155 (2011).
Lee, K.K., Ohyama, T., Yajima, N., Tsubuki, S. & Yonehara, S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276, 19276–19285 (2001).
Tuttle, R.L. et al. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 7, 1133–1137 (2001).
Bernal-Mizrachi, E., Wen, W., Stahlhut, S., Welling, C.M. & Permutt, M.A. Islet beta cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 108, 1631–1638 (2001).
Yuan, Z. et al. Phosphoinositide 3-kinase/Akt inhibits MST1-mediated pro-apoptotic signaling through phosphorylation of threonine 120. J. Biol. Chem. 285, 3815–3824 (2010).
Cinar, B. et al. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J. 26, 4523–4534 (2007).
Trümper, K. et al. Integrative mitogenic role of protein kinase B/Akt in beta-cells. Ann. NY Acad. Sci. 921, 242–250 (2000).
Matallanas, D. et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol. Cell 27, 962–975 (2007).
Valis, K. et al. Hippo/Mst1 stimulates transcription of the proapoptotic mediator NOXA in a FoxO1-dependent manner. Cancer Res. 71, 946–954 (2011).
Grunnet, L.G. et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 58, 1807–1815 (2009).
Opferman, J.T. & Korsmeyer, S.J. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 4, 410–415 (2003).
Yamamoto, S. et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J. Clin. Invest. 111, 1463–1474 (2003).
Lei, K. & Davis, R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 100, 2432–2437 (2003).
Rahmani, M. et al. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood 114, 4507–4516 (2009).
Humphrey, R.K., Yu, S.M., Flores, L.E. & Jhala, U.S. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J. Biol. Chem. 285, 3406–3416 (2010).
Kawamori, D. et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 281, 1091–1098 (2006).
McCulloch, L.J. et al. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol. Genet. Metab. 104, 648–653 (2011).
Claiborn, K.C. et al. Pcif1 modulates Pdx1 protein stability and pancreatic beta cell function and survival in mice. J. Clin. Invest. 120, 3713–3721 (2010).
Miller, M.L. et al. Linear motif atlas for phosphorylation-dependent signaling. Sci. Signal. 1, ra2 (2008).
Frogne, T., Sylvestersen, K.B., Kubicek, S., Nielsen, M.L. & Hecksher-Sorensen, J. Pdx1 is post-translationally modified in vivo and serine 61 is the principal site of phosphorylation. PLoS ONE 7, e35233 (2012).
Dunning, B.E. & Gerich, J.E. The role of alpha cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr. Rev. 28, 253–283 (2007).
Li, Z., Karlsson, F.A. & Sandler, S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low-dose streptozotocin administration in mice. J. Endocrinol. 165, 93–99 (2000).
Lin, Y., Khokhlatchev, A., Figeys, D. & Avruch, J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J. Biol. Chem. 277, 47991–48001 (2002).
Del Re, D.P. et al. Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice. J. Clin. Invest. 120, 3555–3567 (2010).
Graves, J.D., Draves, K.E., Gotoh, Y., Krebs, E.G. & Clark, E.A. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J. Biol. Chem. 276, 14909–14915 (2001).
Graves, J.D. et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 (1998).
Song, H. et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. USA 107, 1431–1436 (2010).
Zhou, D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009).
Yun, H.J. et al. Daxx mediates activation-induced cell death in microglia by triggering MST1 signalling. EMBO J. 30, 2465–2476 (2011).
Odashima, M. et al. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ. Res. 100, 1344–1352 (2007).
Assmann, A., Ueki, K., Winnay, J.N., Kadowaki, T. & Kulkarni, R.N. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol. Cell. Biol. 29, 3219–3228 (2009).
Ahlgren, U., Jonsson, J., Jonsson, L., Simu, K. & Edlund, H. beta-cell–specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763–1768 (1998).
Lebrun, P., Montminy, M.R. & Van Obberghen, E. Regulation of the pancreatic duodenal homeobox-1 protein by DNA-dependent protein kinase. J. Biol. Chem. 280, 38203–38210 (2005).
Costes, S. et al. Beta-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes 60, 227–238 (2011).
Butler, P.C., Meier, J.J., Butler, A.E. & Bhushan, A. The replication of beta cells in normal physiology, in disease and for therapy. Nat. Clin. Pract. Endocrinol. Metab. 3, 758–768 (2007).
Choi, J. et al. Mst1-FoxO signaling protects naive T lymphocytes from cellular oxidative stress in mice. PLoS ONE 4, e8011 (2009).
Dong, Y. et al. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J. Immunol. 183, 3865–3872 (2009).
Ueda, Y. et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat. Commun. 3, 1098 (2012).
Soltani, N. et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 108, 11692–11697 (2011).
Sauter, N.S., Schulthess, F.T., Galasso, R., Castellani, L.W. & Maedler, K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet–induced hyperglycemia. Endocrinology 149, 2208–2218 (2008).
Carew, R.M. et al. Deletion of Irs2 causes reduced kidney size in mice: role for inhibition of GSK3β? BMC Dev. Biol. 10, 73 (2010).
Kawano, Y. et al. Loss of Pdk1-Foxo1 signaling in myeloid cells predisposes to adipose tissue inflammation and insulin resistance. Diabetes 61, 1935–1948 (2012).
Schulthess, F.T. et al. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 9, 125–139 (2009).
Maedler, K. et al. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 50, 69–76 (2001).
Herrera, P.L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127, 2317–2322 (2000).
Surwit, R.S., Kuhn, C.M., Cochrane, C., McCubbin, J.A. & Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37, 1163–1167 (1988).
Kang, H.C. & Bae, Y.H. Transfection of rat pancreatic islet tissue by polymeric gene vectors. Diabetes Technol. Ther. 11, 443–449 (2009).
Shu, L. et al. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes 57, 645–653 (2008).
Shu, L. et al. TCF7L2 promotes beta cell regeneration in human and mouse pancreas. Diabetologia 55, 3296–3307 (2012).
Tolia, N.H. & Joshua-Tor, L. Strategies for protein coexpression in Escherichia coli. Nat. Methods 3, 55–64 (2006).
Arnoult, D. Apoptosis-associated mitochondrial outer membrane permeabilization assays. Methods 44, 229–234 (2008).
Acknowledgements
This work was supported by the JDRF, the German Research Foundation (Emmy Noether Programm MA4172/1-1), the European Research Council, the German Federal Ministry of Science (Diabetes Competence Network), the European Federation for the Study of Diabetes and University of Bremen Research Funds (all to K.M.). We thank J. Bergemann for excellent technical assistance, G. Dharmadhikari and M. Panse for help with the analyses and G. Rutter and D. Schumann for critical discussion. Human islets were provided through the Juvenile Diabetes Research Foundation award 31-2008-413 (European Consortium for Islet Transplantation Islets for Basic Research Program) and by the Integrated Islet Distribution Program. Human pancreatic sections were obtained from the National Disease Research Interchange, which is supported by the US National Institutes of Health. MST1 and dn-MST1 plasmids and adenoviruses were provided by J. Sadoshima and Y. Maejima (Rutgers New Jersey Medical School), PDX1-WT plasmids and pGEX bacterial expression vector by R. Walther (University of Greifswald), INS-1E cells by C. Wollheim (Lund and Geneva Universities), RIP-Cre mice by P. Herrera (University of Geneva) and A. Mansouri (Max Planck Institute for Biophysical Chemistry), mouse pB.RSV.PDX1-GFP plasmid by I. Leibiger (Karolinska University) and rat insulin-2 promoter plasmid by R. Zinkernagel (University of Zurich). Adenovirus carrying eGFP as a control was provided by A.E. Karlsen (Novo Nordisk A/S).
Author information
Authors and Affiliations
Contributions
A.A. conceived of the project, designed all and performed most of the experiments, analyzed the data and wrote the paper. F. Paroni provided experimental and technical support and analyzed data. Z.A., S.K., V.K. and T.Y. performed experiments and analyzed data. T.F. provided mutated PDX1 plasmids, W.T. provided Mst1−/− and Mst1fl/fl mice and J.K.C., F. Pattou and J.O. isolated human islets. K.M. supervised the project and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 8413 kb)
Rights and permissions
About this article
Cite this article
Ardestani, A., Paroni, F., Azizi, Z. et al. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med 20, 385–397 (2014). https://doi.org/10.1038/nm.3482
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3482
This article is cited by
-
Discovery of IHMT-MST1-39 as a novel MST1 kinase inhibitor and AMPK activator for the treatment of diabetes mellitus
Signal Transduction and Targeted Therapy (2023)
-
Pathological roles of bone marrow adipocyte-derived monocyte chemotactic protein-1 in type 2 diabetic mice
Cell Death Discovery (2023)
-
Pharmacological and mechanistic study of PS1, a Pdia4 inhibitor, in β-cell pathogenesis and diabetes in db/db mice
Cellular and Molecular Life Sciences (2023)
-
Insights on the role of anti-inflammatory and immunosuppressive agents in the amelioration of diabetes
Diabetology International (2023)
-
MST1 deletion protects β-cells in a mouse model of diabetes
Nutrition & Diabetes (2022)