Abstract
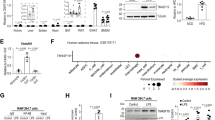
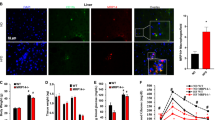
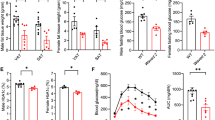
During obesity, macrophage accumulation in adipose tissue propagates the chronic inflammation and insulin resistance associated with type 2 diabetes. The factors, however, that regulate the accrual of macrophages in adipose tissue are not well understood. Here we show that the neuroimmune guidance cue netrin-1 is highly expressed in obese but not lean adipose tissue of humans and mice, where it directs the retention of macrophages. Netrin-1, whose expression is induced in macrophages by the saturated fatty acid palmitate, acts via its receptor Unc5b to block their migration. In a mouse model of diet-induced obesity, we show that adipose tissue macrophages exhibit reduced migratory capacity, which can be restored by blocking netrin-1. Furthermore, hematopoietic deletion of Ntn1 facilitates adipose tissue macrophage emigration, reduces inflammation and improves insulin sensitivity. Collectively, these findings identify netrin-1 as a macrophage retention signal in adipose tissue during obesity that promotes chronic inflammation and insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
14 March 2014
In the version of this article initially published online, the corresponding author’s e-mail address was incorrect. The correct address is kathryn.moore@nyumc.org. The error has been corrected for all versions of this article.
References
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Kintscher, U. et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler. Thromb. Vasc. Biol. 28, 1304–1310 (2008).
Liu, J. et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 (2009).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Weisberg, S.P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Wu, H. et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115, 1029–1038 (2007).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Hotamisligil, G.S., Shargill, N.S. & Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Weisberg, S.P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Arkan, M.C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Koppaka, S. et al. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes 62, 1843–1854 (2013).
Di Gregorio, G.B. et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54, 2305–2313 (2005).
Lumeng, C.N., Bodzin, J.L. & Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Lumeng, C.N., Deyoung, S.M., Bodzin, J.L. & Saltiel, A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 (2007).
Takahashi, K. et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J. Biol. Chem. 278, 46654–46660 (2003).
Chakrabarti, S.K. et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 300, E175–E187 (2011).
Kosteli, A. et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Invest. 120, 3466–3479 (2010).
Odegaard, J.I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Suzuki, K., Kumanogoh, A. & Kikutani, H. Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 9, 17–23 (2008).
Cirulli, V. & Yebra, M. Netrins: beyond the brain. Nat. Rev. Mol. Cell Biol. 8, 296–306 (2007).
Wu, J.Y. et al. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 410, 948–952 (2001).
Takamatsu, H. & Kumanogoh, A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 33, 127–135 (2012).
Shimizu, I. et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab. 18, 491–504 (2013).
Srinivasan, K., Strickland, P., Valdes, A., Shin, G.C. & Hinck, L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell 4, 371–382 (2003).
Salminen, M., Meyer, B.I., Bober, E. & Gruss, P. Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development 127, 13–22 (2000).
Nguyen, A. & Cai, H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc. Natl. Acad. Sci. USA 103, 6530–6535 (2006).
Wilson, B.D. et al. Netrins promote developmental and therapeutic angiogenesis. Science 313, 640–644 (2006).
Arakawa, H. Netrin-1 and its receptors in tumorigenesis. Nat. Rev. Cancer 4, 978–987 (2004).
Fitamant, J. et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 105, 4850–4855 (2008).
Rosenberger, P. et al. Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 10, 195–202 (2009).
van Gils, J.M. et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat. Immunol. 13, 136–143 (2012).
Suganami, T., Nishida, J. & Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arterioscler. Thromb. Vasc. Biol. 25, 2062–2068 (2005).
Boden, G. Interaction between free fatty acids and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 5, 545–549 (2002).
Uysal, K.T., Wiesbrock, S.M., Marino, M.W. & Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610–614 (1997).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Oh, D.Y., Morinaga, H., Talukdar, S., Bae, E.J. & Olefsky, J.M. Increased macrophage migration into adipose tissue in obese mice. Diabetes 61, 346–354 (2012).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Feig, J.E. et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J. Clin. Invest. 120, 4415–4424 (2010).
Kováčiková, M. et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int. J. Obes. (Lond) 35, 91–98 (2011).
Wang, Q. et al. Differential effect of weight loss with low-fat diet or high-fat diet restriction on inflammation in the liver and adipose tissue of mice with diet-induced obesity. Atherosclerosis 219, 100–108 (2011).
Jung, D.Y. et al. Short-term weight loss attenuates local tissue inflammation and improves insulin sensitivity without affecting adipose inflammation in obese mice. Am. J. Physiol. Endocrinol. Metab. 304, E964–E976 (2013).
Diehl, G.E. et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX3CR1hi cells. Nature 494, 116–120 (2013).
Trogan, E. et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl. Acad. Sci. USA 103, 3781–3786 (2006).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Suganami, T. et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 27, 84–91 (2007).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolismin adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Wang, W., Reeves, W.B., Pays, L., Mehlen, P. & Ramesh, G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am. J. Pathol. 175, 1010–1018 (2009).
Mirakaj, V. et al. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am. J. Respir. Crit. Care Med. 181, 815–824 (2010).
Ly, N.P. et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 102, 14729–14734 (2005).
Tadagavadi, R.K., Wang, W. & Ramesh, G. Netrin-1 regulates TH1/TH2/TH17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J. Immunol. 185, 3750–3758 (2010).
Ranganathan, P.V., Jayakumar, C., Mohamed, R., Dong, Z. & Ramesh, G. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2–mediated PGE2 production. Kidney Int. 83, 1087–1098 (2013).
Khan, J.A. et al. Systemic human netrin-1 gene delivery by adeno-associated virus type 8 alters leukocyte accumulation and atherogenesis in vivo. Gene Ther. 18, 437–444 (2011).
Dominguez, H. et al. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 42, 517–525 (2005).
Larsen, C.M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Lin, Y. et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J. Biol. Chem. 280, 4617–4626 (2005).
Yeop Han, C. et al. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes 59, 386–396 (2010).
Bachman, E.S. et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 (2002).
Könner, A.C. et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5, 438–449 (2007).
Acknowledgements
Support for this work came from the US National Institutes of Health (RC1HL100815 to K.J.M. and T32HL098129 to E.J.H.), the American Heart Association (13POST14490016 to B.R.) and the Canadian Institutes of Health Research (MOP-2390941 to R.M.).
Author information
Authors and Affiliations
Contributions
B.R., K.J.R. and K.J.M. conceived of the study; B.R. performed the in vivo studies and data analyses, and assisted in the preparation of the manuscript; E.J.H., W.S. and S.H. assisted with mouse studies; F.J.S. performed ELISA assays; M.M. assisted with flow cytometry studies, S.O. performed RT-PCR; G.M. and R.M. collected human samples; K.J.R. and M.G. performed serum netrin-1 ELISA; T.D.R. performed western blotting; A.W. performed migration assays; and K.J.M. designed, analyzed and interpreted the studies, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1–2 (PDF 1493 kb)
Rights and permissions
About this article
Cite this article
Ramkhelawon, B., Hennessy, E., Ménager, M. et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med 20, 377–384 (2014). https://doi.org/10.1038/nm.3467
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3467
This article is cited by
-
Macrophage function in adipose tissue homeostasis and metabolic inflammation
Nature Immunology (2023)
-
Obesity and prostate cancer — microenvironmental roles of adipose tissue
Nature Reviews Urology (2023)
-
Netrin-1 and RGMa: Novel Regulators of Atherosclerosis-Related Diseases
Cardiovascular Drugs and Therapy (2023)
-
Urinary and circulatory netrin-1 as biomarker in diabetes and its related complications: a systematic review and meta-analysis
Endocrine (2023)
-
Netrin-1 blockade inhibits tumor associated Myeloid-derived suppressor cells, cancer stemness and alleviates resistance to chemotherapy and immune checkpoint inhibitor
Cell Death & Differentiation (2023)