Abstract
Podocytes are critical in the maintenance of a healthy glomerular filter; however, they have been difficult to study in the intact kidney because of technical limitations. Here we report the development of serial multiphoton microscopy (MPM) of the same glomeruli over several days to visualize the motility of podocytes and parietal epithelial cells (PECs) in vivo. In podocin-GFP mice, podocytes formed sporadic multicellular clusters after unilateral ureteral ligation and migrated into the parietal Bowman's capsule. The tracking of single cells in podocin-confetti mice featuring cell-specific expression of CFP, GFP, YFP or RFP revealed the simultaneous migration of multiple podocytes. In phosphoenolpyruvate carboxykinase (PEPCK)-GFP mice, serial MPM found PEC-to-podocyte migration and nanotubule connections. Our data support a highly dynamic rather than a static nature of the glomerular environment and cellular composition. Future application of this new approach should advance our understanding of the mechanisms of glomerular injury and regeneration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Patrakka, J. & Tryggvason, K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 5, 463–468 (2009).
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Huber, T.B. et al. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J. Clin. Invest. 116, 1337–1345 (2006).
Jones, N. et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 (2006).
Kaplan, J.M. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Kestilä, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Mundel, P. & Reiser, J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 77, 571–580 (2010).
Reiser, J. et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and α3 integrin. J. Biol. Chem. 279, 34827–34832 (2004).
Shih, N.-Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Winn, M.P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Peti-Peterdi, J., Burford, J.L. & Hackl, M.J. The first decade of using multiphoton microscopy for high-power kidney imaging. Am. J. Physiol. Renal Physiol. 302, F227–F233 (2012).
Devi, S. et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med. 19, 107–112 (2013).
Peti-Peterdi, J. & Sipos, A. A high-powered view of the filtration barrier. J. Am. Soc. Nephrol. 21, 1835–1841 (2010).
Schießl, I.M., Bardehle, S. & Castrop, H. Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS ONE 8, e52499 (2013).
Muzumdar, M.D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Snippert, H.J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Grgic, I. et al. Imaging of podocyte foot processes by fluorescence microscopy. J. Am. Soc. Nephrol. 23, 785–791 (2012).
Höhne, M. et al. Light microscopic visualization of podocyte ultrastructure demonstrates oscillating glomerular contractions. Am. J. Pathol. 182, 332–338 (2013).
Khoury, C.C. et al. Visualizing the mouse podocyte with multiphoton microscopy. Biochem. Biophys. Res. Commun. 427, 525–530 (2012).
Moeller, M.J., Sanden, S.K., Soofi, A., Wiggins, R.C. & Holzman, L.B. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35, 39–42 (2003).
Wang, J. et al. Tamoxifen-inducible podocyte-specific iCre recombinase transgenic mouse provides a simple approach for modulation of podocytes in vivo. Genesis 48, 446–451 (2010).
Shankland, S.J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69, 2131–2147 (2006).
D'Agati, V.D., Kaskel, F.J. & Falk, R.J. Focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 2398–2411 (2011).
Moeller, M.J. et al. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J. Am. Soc. Nephrol. 15, 61–67 (2004).
Appel, D. et al. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 20, 333–343 (2009).
Ronconi, E. et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 20, 322–332 (2009).
Chevalier, R.L., Forbes, M.S. & Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145–1152 (2009).
Forbes, M.S., Thornhill, B.A. & Chevalier, R.L. Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: a new look at an old model. Am. J. Physiol. Renal Physiol. 301, F110–F117 (2011).
Dai, C., Saleem, M.A., Holzman, L.B., Mathieson, P. & Liu, Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 77, 962–973 (2010).
Rankin, E.B., Tomaszewski, J.E. & Haase, V.H. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 66, 2576–2583 (2006).
Nakano, D. et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J. Am. Soc. Nephrol. 23, 1847–1856 (2012).
Salmon, A.H.J. et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J. Am. Soc. Nephrol. 23, 1339–1350 (2012).
Kang, J.J., Toma, I., Sipos, A., McCulloch, F. & Peti-Peterdi, J. Quantitative imaging of basic functions in renal (patho)physiology. Am. J. Physiol. Renal Physiol. 291, F495–F502 (2006).
Smeets, B. et al. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J. Am. Soc. Nephrol. 20, 2604–2615 (2009).
Benigni, A. et al. Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am. J. Pathol. 179, 628–638 (2011).
Gibson, I.W., Downie, T.T., More, I.A. & Lindop, G.B. Atubular glomeruli and glomerular cysts—a possible pathway for nephron loss in the human kidney? J. Pathol. 179, 421–426 (1996).
Bariety, J., Mandet, C., Hill, G.S. & Bruneval, P. Parietal podocytes in normal human glomeruli. J. Am. Soc. Nephrol. 17, 2770–2780 (2006).
Schepers, A.G. et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735 (2012).
Rustom, A., Saffrich, R., Markovic, I., Walther, P. & Gerdes, H.-H. Nanotubular highways for intercellular organelle transport. Science 303, 1007–1010 (2004).
Acknowledgements
This work was supported in part by US National Institutes of Health grant DK64324, American Diabetes Association grant 1-11-BS-121 and the University Kidney Research Organization to J.P.-P., Deutsche Forschungsgemeinschaft grant SFB635 to T.B. and US National Institutes of Health grant DK076077 to K.S. M.J.H. was supported by Deutsche Forschungsgemeinschaft grant HA-6212. We thank M. Hammerschmidt and H.-M. Pogoda (Institute of Developmental Biology, University of Cologne) for granting access to their imaging facility. We thank F.R. Danesh (Baylor College of Medicine) and V. Haase (Vanderbilt University) for providing podocin-iCreERT2 and PEPCK-Cre mice, respectively.
Author information
Authors and Affiliations
Contributions
J.P.-P. conceived the study. J.P.-P., M.J.H. and J.L.B. designed the experiments, coordinated the study and analyzed the data. J.P.-P., M.J.H. and T.B. wrote the manuscript. M.J.H., J.L.B., L.L., K.V., B.S. and K.S. performed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1016 kb)
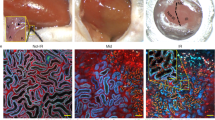
Optical sectioning of a glomerulus in a control Pod-GFP mouse in vivo using MPM imaging.
Z-sections of the same glomerulus are shown on three panels demonstrating native GFP fluorescence in podocytes (green) and Tomato fluorescence in all other cells (red, left panel) before iv injection of Alexa594-albumin (red) to label the intravascular space (middle panel). Circulating red blood cells appear as dark objects within capillaries. Lucifer Yellow, a freely filtered dye was injected iv (right panel) to label the Bowman's space. Note the normal glomerular anatomy and the high structural detail provided by MPM imaging including the clear view of the glomerulo-tubular junction and the apical (brush border) membrane of the proximal tubule (in negative due to lack of LY labeling). (AVI 6241 kb)
Optical sectioning of a glomerulus in a Pod-GFP mouse kidney in vivo 5 weeks after UUO.
Podocyte (green) clusters at the urinary pole of a glomerulus form connections and migrate to the parietal Bowman's capsule. These podocyte projections then invade the PEC layer and the remainder of the proximal tubule fragment. The same glomerulus is shown in Supplementary Figure 2a. (AVI 3182 kb)
Optical sectioning of a glomerulus in a Pod-GFP mouse kidney in vivo 4 days after adriamycin treatment.
While most parts of the glomerular filtration barrier show normal morphology including primary and secondary podocyte processes, a large podocyte (green) cluster is visible in the mid-portion of the glomerular tuft. There is no evidence for the presence of GFP+ parietal cells in the Bowman's capsule. Plasma is labeled red with Alexa594-albumin. The same glomerulus is shown in Figure 2d. (AVI 3043 kb)
Serial in vivo MPM imaging of the same glomerulus in Pod-GFP mice after UUO, once in every 24 hours.
Optical sections (Z-stack) of the same glomerulus are shown at different time points as indicated. Podocytes (green) from a hypercellular area at the urinary pole appear to migrate away from the capillary tuft to form a projection into the remainder of the proximal tubule. Alexa594-albumin (red) labels the circulating plasma. The red unctuate fluorescence in the interstitium is due to endocytosed Alexa594-albumin. The same glomerulus is shown in Figure 3a-b. (AVI 6184 kb)
Serial in vivo MPM imaging of the same glomerulus in Pod-GFP mice after UUO, at baseline and 24 hours
Optical sections (Z-stack) of the same glomerulus are shown at different time points as indicated. The increased size (growth) of parietal GFP+ cell projections (green) is visible 24 hours after the previous imaging session. The arrows point to parietal regions with continuous GFP+ cell coverage, in contrast to non-continuous GFP+ coverage at same locations 24 hours prior. Plasma is labeled red with Alexa594-albumin. The same glomerulus is shown in Figure 3c-d. (MP4 553 kb)
Optical sectioning of a glomerulus in the multi-color Pod-Confetti mouse model in vivo using MPM.
Podocytes appeared to be labeled random in one of the four colors by expressing either membrane-targeted CFP, nuclear GFP, cytosolic YFP or cytosolic RFP. Overlay of the 70 kDa Texas red dextran (plasma dye) image was converted to grayscale to show tissue morphology and blood flow. The same glomerulus is shown in Figure 4a. (AVI 5645 kb)
Serial in vivo MPM imaging of the same glomerulus in the multi-color Pod-Confetti mouse after UUO at baseline and 24 hours later
The pair of Z-stack images (movie of optical sections) provide visual evidence for the appearance of a new visceral podocyte. Compared to 24h prior (left panel) a new, nuclear GFP-labeled podocyte appeared around a glomerular capillary (right panel) as indicated in Figure 4d-e. (AVI 2874 kb)
Serial in vivo MPM imaging of the same glomerulus in the PEPCK-GFP mouse model after UUO, once in every 24 hours.
Optical sections (Z-stack) of the same glomerulus are shown at different time points as indicated. A GFP-expressing PEC (green) at the vascular pole of the glomerulus forms projections propagating into both the visceral and parietal layers. The length of the green projections increased between the time points of 0, 24, and 48 h. The same glomerulus is shown in Figure 5b-c. (AVI 7908 kb)
Time-lapse in vivo MPM imaging of nanotubules in the PEPCK-GFP mouse model after UUO.
Nanotubules (green) are visualized in the intact glomerulus connecting the PEC layer with the glomerular tuft. The position of the stretched nanotubules appears to be steady during the two minutes of continuous imaging. The same glomerulus is shown in Figure 5d. (AVI 4912 kb)
Rights and permissions
About this article
Cite this article
Hackl, M., Burford, J., Villanueva, K. et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med 19, 1661–1666 (2013). https://doi.org/10.1038/nm.3405
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3405
This article is cited by
-
Kidney lipid dysmetabolism and lipid droplet accumulation in chronic kidney disease
Nature Reviews Nephrology (2023)
-
Immune cell behaviour and dynamics in the kidney — insights from in vivo imaging
Nature Reviews Nephrology (2022)
-
Intravital microscopy imaging of kidney injury and regeneration
Renal Replacement Therapy (2021)
-
Advances in fluorescence microscopy techniques to study kidney function
Nature Reviews Nephrology (2021)
-
Endothelium structure and function in kidney health and disease
Nature Reviews Nephrology (2019)