Abstract
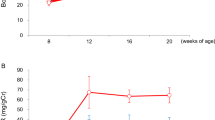
The molecular link between proteinuria and hyperlipidemia in nephrotic syndrome is not known. We show in the present study that plasma angiopoietin-like 4 (Angptl4) links proteinuria with hypertriglyceridemia through two negative feedback loops. In previous studies in a rat model that mimics human minimal change disease, we observed localized secretion by podocytes of hyposialylated Angptl4, a pro-proteinuric form of the protein. But in this study we noted high serum levels of Angptl4 (presumably normosialylated based on a neutral isoelectric point) in other glomerular diseases as well. Circulating Angptl4 was secreted by extrarenal organs in response to an elevated plasma ratio of free fatty acids (FFAs) to albumin when proteinuria reached nephrotic range. In a systemic feedback loop, these circulating pools of Angptl4 reduced proteinuria by interacting with glomerular endothelial αvβ5 integrin. Blocking the Angptl4–β5 integrin interaction or global knockout of Angptl4 or β5 integrin delayed recovery from peak proteinuria in animal models. But at the same time, in a local feedback loop, the elevated extrarenal pools of Angptl4 reduced tissue FFA uptake in skeletal muscle, heart and adipose tissue, subsequently resulting in hypertriglyceridemia, by inhibiting lipoprotein lipase (LPL)-mediated hydrolysis of plasma triglycerides to FFAs. Injecting recombinant human ANGPTL4 modified at a key LPL interacting site into nephrotic Buffalo Mna and Zucker Diabetic Fatty rats reduced proteinuria through the systemic loop but, by bypassing the local loop, without increasing plasma triglyceride levels. These data show that increases in circulating Angptl4 in response to nephrotic-range proteinuria reduces the degree of this pathology, but at the cost of inducing hypertriglyceridemia, while also suggesting a possible therapy to treat these linked pathologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vaziri, N.D. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 63, 1964–1976 (2003).
Marsh, J.B. & Drabkin, D.L. Experimental reconstruction of metabolic pattern of lipid nephrosis: key role of hepatic protein synthesis in hyperlipemia. Metabolism 9, 946–955 (1960).
Merkel, M., Eckel, R.H. & Goldberg, I.J. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 43, 1997–2006 (2002).
Weinstock, P.H. et al. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J. Clin. Invest. 96, 2555–2568 (1995).
Shearer, G.C. & Kaysen, G.A. Endothelial bound lipoprotein lipase (LpL) depletion in hypoalbuminemia results from decreased endothelial binding, not decreased secretion. Kidney Int. 70, 647–653 (2006).
Ghiggeri, G.M. et al. Characterization of cationic albumin in minimal change nephropathy. Kidney Int. 32, 547–553 (1987).
Clement, L.C. et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med. 17, 117–122 (2011).
Chugh, S.S., Clement, L.C. & Macé, C. New insights into human minimal change disease: lessons from animal models. Am. J. Kidney Dis. 59, 284–292 (2012).
Sukonina, V., Lookene, A., Olivecrona, T. & Olivecrona, G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA 103, 17450–17455 (2006).
Yoshida, K., Shimizugawa, T., Ono, M. & Furukawa, H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43, 1770–1772 (2002).
Romeo, S. et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39, 513–516 (2007).
Yin, W. et al. Genetic variation in ANGPTL4 provides insights into protein processing and function. J. Biol. Chem. 284, 13213–13222 (2009).
Clement, L.C. et al. Early changes in gene expression that influence the course of primary glomerular disease. Kidney Int. 72, 337–347 (2007).
Liu, G., Clement, L., Kanwar, Y.S., Avila-Casado, C. & Chugh, S.S. ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J. Biol. Chem. 281, 39681–39692 (2006).
Nakamura, T. et al. Sclerotic lesions in the glomeruli of Buffalo/Mna rats. Nephron 43, 50–55 (1986).
Le Berre, L. et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J. Clin. Invest. 109, 491–498 (2002).
Neuger, L. et al. Effects of heparin on the uptake of lipoprotein lipase in rat liver. BMC Physiol. 4, 13 (2004).
Chang, S.F., Reich, B., Brunzell, J.D. & Will, H. Detailed characterization of the binding site of the lipoprotein lipase-specific monoclonal antibody 5D2. J. Lipid Res. 39, 2350–2359 (1998).
Nagase, S., Shimamune, K. & Shumiya, S. Albumin-deficient rat mutant. Science 205, 590–591 (1979).
Kikuchi, H., Tamura, S., Nagase, S. & Tsuiki, S. Hypertriacylglycerolemia and adipose tissue lipoprotein lipase activity in the Nagase analbuminemic rat. Biochim. Biophys. Acta 744, 165–170 (1983).
Kersten, S. et al. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 29, 969–974 (2009).
Staiger, H. et al. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-δ and is of metabolic relevance in humans. Diabetes 58, 579–589 (2009).
Georgiadi, A. et al. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor β/δ and protects against fatty acid–induced oxidative stress. Circ. Res. 106, 1712–1721 (2010).
Zhu, P. et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2−:H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 19, 401–415 (2011).
Mandard, S. et al. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281, 934–944 (2006).
Zilleruelo, G., Hsia, S.L., Freundlich, M., Gorman, H.M. & Strauss, J. Persistence of serum lipid abnormalities in children with idiopathic nephrotic syndrome. J. Pediatr. 104, 61–64 (1984).
Yu, X. et al. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc. Natl. Acad. Sci. USA 102, 1767–1772 (2005).
Avila-Casado, M.C. et al. Proteinuria in rats induced by serum from patients with collapsing glomerulopathy. Kidney Int. 66, 133–143 (2004).
Ferris, M. et al. Patient recruitment into a multicenter randomized clinical trial for kidney disease: report of the focal segmental glomerulosclerosis clinical trial (FSGS CT). Clin. Transl. Sci. 6, 13–20 (2013).
Chugh, S. et al. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int. 59, 601–613 (2001).
Takemoto, M. et al. A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 161, 799–805 (2002).
Liu, G. et al. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J. Clin. Invest. 112, 209–221 (2003).
Bengtsson-Olivecrona, G. & Olivecrona, T. Lipoprotein analysis—a practical approach. in Practical Approach Series (eds. Converse, C.A. & Skinner, E.R.) 169–185 (Oxford University Press, New York, 1992).
Acknowledgements
We thank investigators of the FSGS clinical trial for providing baseline plasma and urine samples from patients with FSGS; M. del Nogal-Avila (University of Alabama at Birmingham (UAB)) for selected real-time PCR studies; H. Donoro (UAB) for assistance with animal colony management; E. Soria (Instituto Nacional de Cardiologia) for immunogold electron microscopy studies; H. Chung (UAB) for advice on LPL assays; V. Kumar (UAB) for help in collecting Institutional Review Board–approved patient sera from transplant patients; D. Salant (Boston University) for γ2-NTS; A. Köster (Eli Lilly) for Angptl4−/− mice; M. Mitsuyama (Kyoto University) for Buffalo Mna rats; J. Brunzell (University of Washington) for 5D2 monoclonal antibody; F. Danesh (MD Anderson Cancer Center) for cultured rat glomerular endothelial cells; UAB–University of California-San Diego George O'Brien Center Core C for measuring urine and serum creatinine by mass spectrometry; UAB Nephrology Research and Training Center for equipment use; and UAB Research Foundation for filing patent PCT/US2011/039255 for the use of ANGPTL4 mutants as therapeutic agents for nephrotic syndrome. This work is supported by the US National Institutes of Health (R01DK077073 and R01DK090035 to S.S.C., K01DK096127 to L.C.C. and T32DK007545 to C.M.).
Author information
Authors and Affiliations
Contributions
L.C.C. maintained transgenic rat colonies and conducted rat experiments, developed stable cell lines and conducted imaging studies and selected gene expression studies. C.M. maintained the Itgb5−/− mouse colony and conducted mouse studies, did assays for Angptl4, V5-tagged proteins, triglycerides and free fatty acids and performed two-dimensional gel studies, western blotting, selected gene expression studies, protein interaction studies and albumin depletion studies. C.A.-C. interpreted and analyzed light microscopy, electron microscopy and immunogold electron microscopy studies. J.A.J. obtained blood and tissue from Nagase rats and provided useful advice on Nagase rat biology. S.K. conducted experiments with Angptl4−/− mice and made substantial contributions to the preparation and revision of the manuscript. S.S.C. acted as senior investigator, planned and supervised the study, generated mutant ANGPTL4 constructs to develop stable cell lines, conducted selected gene expression and animal studies and wrote and revised the manuscript with input from other authors.
Corresponding author
Ethics declarations
Competing interests
S.S.C. is founder, president and chief executive officer of GDTHERAPY LLC and has filed patents related to the use of ANGPTL4 mutants (PCT/US2011/039255) and precursors of sialic acid, including ManNAc (PCT/US2011/039058), for the treatment of nephrotic syndrome. S.S.C. may benefit financially from these patents in the future.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–6 (PDF 9229 kb)
Rights and permissions
About this article
Cite this article
Clement, L., Macé, C., Avila-Casado, C. et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med 20, 37–46 (2014). https://doi.org/10.1038/nm.3396
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3396
This article is cited by
-
Dual role of ANGPTL4 in inflammation
Inflammation Research (2023)
-
Circulating ANGPTL8 levels and risk of kidney function decline: Results from the 4C Study
Cardiovascular Diabetology (2021)
-
The role of the immune system in idiopathic nephrotic syndrome
Molecular and Cellular Pediatrics (2021)
-
Utility of non-HDL-C in predicting proteinuria remission of idiopathic membranous nephropathy: a retrospective cohort study
Lipids in Health and Disease (2021)
-
Immunopathogenesis of idiopathic nephrotic syndrome in children: two sides of the coin
World Journal of Pediatrics (2021)