Abstract
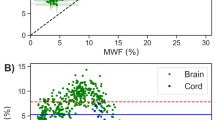
Here, we describe a quantitative neuroimaging method to estimate the macromolecular tissue volume (MTV), a fundamental measure of brain anatomy. By making measurements over a range of field strengths and scan parameters, we tested the key assumptions and the robustness of the method. The measurements confirm that a consistent quantitative estimate of MTV can be obtained across a range of scanners. MTV estimates are sufficiently precise to enable a comparison between data obtained from an individual subject with control population data. We describe two applications. First, we show that MTV estimates can be combined with T1 and diffusion measurements to augment our understanding of the tissue properties. Second, we show that MTV provides a sensitive measure of disease status in individual patients with multiple sclerosis. The MTV maps are obtained using short clinically appropriate scans that can reveal how tissue changes influence behavior and cognition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tofts, P. Quantitative MRI of the Brain Measuring Changes Caused by Disease (John Wiley & Sons, Chichester, West Sussex; Hoboken, NJ, 2003).
Laule, C. et al. Magnetic resonance imaging of myelin. Neurotherapeutics 4, 460–484 (2007).
Deoni, S.C. Magnetic resonance relaxation and quantitative measurement in the brain. Methods Mol. Biol. 711, 65–108 (2011).
Alexander, A.L. et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging atains. Brain Connect. 1, 423–426 (2011).
Fatouros, P.P. & Marmarou, A. Use of magnetic resonance imaging for in vivo measurements of water content in human brain: method and normal values. J. Neurosurg. 90, 109–115 (1999).
Laule, C. et al. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J. Neurol. 251, 284–293 (2004).
Neeb, H., Zilles, K. & Shah, N.J. A new method for fast quantitative mapping of absolute water content in vivo. Neuroimage 31, 1156–1168 (2006).
Ashburner, J. & Friston, K.J. Voxel-based morphometry–the methods. Neuroimage 11, 805–821 (2000).
Fischl, B. & Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 97, 11050–11055 (2000).
Gogtay, N. et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 101, 8174–8179 (2004).
Kakeda, S. & Korogi, Y. The efficacy of a voxel-based morphometry on the analysis of imaging in schizophrenia, temporal lobe epilepsy, and Alzheimer's disease/mild cognitive impairment: a review. Neuroradiology 52, 711–721 (2010).
Kanai, R. & Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 12, 231–242 (2011).
May, A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn. Sci. 15, 475–482 (2011).
Thomas, C. & Baker, C.I. Remodeling human cortex through training: comment on May. Trends Cogn. Sci. 16, 96–97 (2012).
Norton, W.T. & Autilio, L.A. The lipid composition of purified bovine brain myelin. J. Neurochem. 13, 213–222 (1966).
Bottomley, P.A., Foster, T.H., Argersinger, R.E. & Pfeifer, L.M. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1–100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med. Phys. 11, 425–448 (1984).
Mansfield, P. & Morris, P.G. NMR Imaging in Biomedicine (Academic Press, London, 1982).
Rooney, W.D. et al. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Reson. Med. 57, 308–318 (2007).
Fram, E.K. et al. Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn. Reson. Imaging 5, 201–208 (1987).
Koenig, S.H. Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn. Reson. Med. 20, 285–291 (1991).
Kucharczyk, W., Macdonald, P.M., Stanisz, G.J. & Henkelman, R.M. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology 192, 521–529 (1994).
Aboitiz, F., Scheibel, A.B., Fisher, R.S. & Zaidel, E. Fiber composition of the human corpus callosum. Brain Res. 598, 143–153 (1992).
Barazany, D., Basser, P.J. & Assaf, Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. Brain 132, 1210–1220 (2009).
Stikov, N. et al. Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. Neuroimage 54, 1112–1121 (2011).
Alexander, A.L., Lee, J.E., Lazar, M. & Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329 (2007).
Beaulieu, C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 15, 435–455 (2002).
Paus, T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 72, 26–35 (2010).
Yeatman, J.D., Dougherty, R.F., Myall, N.J., Wandell, B.A. & Feldman, H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS ONE 7, e49790 (2012).
Wedeen, V.J. et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41, 1267–1277 (2008).
Gelman, N., Ewing, J.R., Gorell, J.M., Spickler, E.M. & Solomon, E.G. Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: relation to estimated iron and water contents. Magn. Reson. Med. 45, 71–79 (2001).
Does, M.D. & Gore, J.C. Compartmental study of T(1) and T(2) in rat brain and trigeminal nerve in vivo. Magn. Reson. Med. 47, 274–283 (2002).
Filippi, M. & Rocca, M.A. MR imaging of multiple sclerosis. Radiology 259, 659–681 (2011).
Lövblad, K.O. et al. MR imaging in multiple sclerosis: review and recommendations for current practice. AJNR Am. J. Neuroradiol. 31, 983–989 (2010).
Poloni, G., Minagar, A., Haacke, E.M. & Zivadinov, R. Recent developments in imaging of multiple sclerosis. Neurologist 17, 185–204 (2011).
MacKay, A.L. et al. MR relaxation in multiple sclerosis. Neuroimaging Clin. N. Am. 19, 1–26 (2009).
Popescu, B.F. & Lucchinetti, C.F. Pathology of demyelinating diseases. Annu. Rev. Pathol. 7, 185–217 (2012).
Le Bihan, D. et al. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging 13, 534–546 (2001).
Glasser, M.F. & Van Essen, D.C. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 31, 11597–11616 (2011).
Noterdaeme, O., Anderson, M., Gleeson, F. & Brady, S.M. Intensity correction with a pair of spoiled gradient recalled echo images. Phys. Med. Biol. 54, 3473–3489 (2009).
Volz, S., Noth, U. & Deichmann, R. Correction of systematic errors in quantitative proton density mapping. Magn. Reson. Med. 68, 74–85 (2012).
Koenig, B.W. & Gawrisch, K. Specific volumes of unsaturated phosphatidylcholines in the liquid crystalline lamellar phase. Biochim. Biophys. Acta 1715, 65–70 (2005).
Loosley-Millman, M.E., Rand, R.P. & Parsegian, V.A. Effects of monovalent ion binding and screening on measured electrostatic forces between charged phospholipid bilayers. Biophys. J. 40, 221–232 (1982).
Ulrich, A.S. & Watts, A. Molecular response of the lipid headgroup to bilayer hydration monitored by 2H-NMR. Biophys. J. 66, 1441–1449 (1994).
Nagle, J.F. Theory of the main lipid bilayer phase transition. Annu. Rev. Phys. Chem. 31, 157–196 (1980).
Polman, C.H. et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 58, 840–846 (2005).
Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33, 1444–1452 (1983).
Barral, J.K. et al. A robust methodology for in vivo T1 mapping. Magn. Reson. Med. 64, 1057–1067 (2010).
Chang, L.C., Koay, C.G., Basser, P.J. & Pierpaoli, C. Linear least-squares method for unbiased estimation of T1 from SPGR signals. Magn. Reson. Med. 60, 496–501 (2008).
Dale, A.M., Fischl, B. & Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999).
Hopkins, A.L., Yeung, H.N. & Bratton, C.B. Multiple field strength in vivo T1 and T2 for cerebrospinal fluid protons. Magn. Reson. Med. 3, 303–311 (1986).
Sigalovsky, I.S., Fischl, B. & Melcher, J.R. Mapping an intrinsic MR property of gray matter in auditory cortex of living humans: a possible marker for primary cortex and hemispheric differences. Neuroimage 32, 1524–1537 (2006).
Yarnykh, V.L. & Yuan, C. Cross-relaxation imaging reveals detailed anatomy of white matter fiber tracts in the human brain. Neuroimage 23, 409–424 (2004).
Basser, P.J., Pajevic, S., Pierpaoli, C., Duda, J. & Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632 (2000).
Mori, S., Crain, B.J., Chacko, V.P. & van Zijl, P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 (1999).
Dougherty, R.F., Ben-Shachar, M., Bammer, R., Brewer, A.A. & Wandell, B.A. Functional organization of human occipital-callosal fiber tracts. Proc. Natl. Acad. Sci. USA 102, 7350–7355 (2005).
Wakana, S., Jiang, H., Nagae-Poetscher, L.M., van Zijl, P.C. & Mori, S. Fiber tract-based atlas of human white matter anatomy. Radiology 230, 77–87 (2004).
Corouge, I., Gouttard, S. & Gerig, G. A statistical shape model of individual fiber tracts extracted from diffusion tensor MRI. Lect. Notes Comput. Sci. 3217, 671–679 (2004).
Acknowledgements
We acknowledge J. Barral, M. Gutman, H. Horiguchi, I. Levesque, A. Sherbondy and A. Takahashi for helpful advice and feedback. We thank S. Phipps, I. Levesque and A. Kerr for help in data analysis and acquisition. This work was supported by the US National Institutes of Health research grants RO1-EY15000 and NSF grant BCS-1228397. A.M. is the recipient of support from the Human Frontier Science Program and a Jewish Community Federation Program Machiah Foundation Fellowship. N.-J.C. is the recipient of support from the Singapore National Research Foundation (NRF-NRFF2011-01).
Author information
Authors and Affiliations
Contributions
A.M. and B.A.W. developed the method, wrote the manuscript and prepared the figures. J.D.Y., N.S., M.L.P., R.F.D., K.B.-P. and A.M. obtained the data. N.-J.C. provided the lipid phantoms. N.S., R.F.D., K.N.K. and A.M. developed analysis tools. J.P. and L.H.H. diagnosed the patient with multiple sclerosis and enabled those scans. A.M. and J.D.Y. developed the diffusion methods and applications. B.A.W. provided equipment and administered the experiment. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Stanford University has filed a US patent application describing the technology used to measure PD, T1, MTV, VIP and SIR in this study (A.M., R.F.D. and B.A.W.).
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figures 1–12, Supplementary Discussion and Supplementary Data. (PDF 3092 kb)
Rights and permissions
About this article
Cite this article
Mezer, A., Yeatman, J., Stikov, N. et al. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med 19, 1667–1672 (2013). https://doi.org/10.1038/nm.3390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3390
This article is cited by
-
Non-invasive assessment of normal and impaired iron homeostasis in the brain
Nature Communications (2023)
-
Comparing retinotopic maps of children and adults reveals a late-stage change in how V1 samples the visual field
Nature Communications (2023)
-
Longitudinal development of category representations in ventral temporal cortex predicts word and face recognition
Nature Communications (2023)
-
Mapping myelin in white matter with T1-weighted/T2-weighted maps: discrepancy with histology and other myelin MRI measures
Brain Structure and Function (2023)
-
White matter myelination during early infancy is linked to spatial gradients and myelin content at birth
Nature Communications (2022)