Abstract
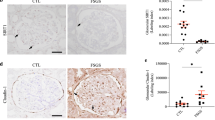
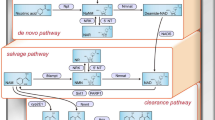
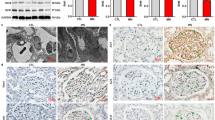
Sirtuin 1 (Sirt1), a NAD+-regulated deacetylase with numerous known positive effects on cellular and whole-body metabolism, is expressed in the renal cortex and medulla. It is known to have protective effects against age-related disease, including diabetes. Here we investigated the protective role of Sirt1 in diabetic renal damage. We found that Sirt1 in proximal tubules (PTs) was downregulated before albuminuria occurred in streptozotocin-induced or obese (db/db) diabetic mice. PT-specific SIRT1 transgenic and Sirt1 knockout mice showed prevention and aggravation of the glomerular changes that occur in diabetes, respectively, and nondiabetic knockout mice exhibited albuminuria, suggesting that Sirt1 in PTs affects glomerular function. Downregulation of Sirt1 and upregulation of the tight junction protein Claudin-1 by SIRT1-mediated epigenetic regulation in podocytes contributed to albuminuria. We did not observe these phenomena in 5/6 nephrectomized mice. We also demonstrated retrograde interplay from PTs to glomeruli using nicotinamide mononucleotide (NMN) from conditioned medium, measurement of the autofluorescence of photoactivatable NMN and injection of fluorescence-labeled NMN. In human subjects with diabetes, the levels of SIRT1 and Claudin-1 were correlated with proteinuria levels. These results suggest that Sirt1 in PTs protects against albuminuria in diabetes by maintaining NMN concentrations around glomeruli, thus influencing podocyte function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Roxburgh, S.A. et al. Allelic depletion of grem1 attenuates diabetic kidney disease. Diabetes 58, 1641–1650 (2009).
Rodgers, J.T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Bordone, L. & Guarente, L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 6, 298–305 (2005).
Purushotham, A. et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 9, 327–338 (2009).
Wang, R.H. et al. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Invest. 121, 4477–4490 (2011).
Moynihan, K.A. et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2, 105–117 (2005).
Kume, S. et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 120, 1043–1055 (2010).
He, W. et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Invest. 120, 1056–1068 (2010).
Kitada, M., Kume, S., Imaizumi, N. & Koya, D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 60, 634–643 (2011).
Tikoo, K., Singh, K., Kabra, D., Sharma, V. & Gaikwad, A. Change in histone H3 phosphorylation, MAP kinase p38, SIR 2 and p53 expression by resveratrol in preventing streptozotocin induced type I diabetic nephropathy. Free Radic. Res. 42, 397–404 (2008).
Kitada, M. et al. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp. Diabetes Res. 2011, 908185 (2011).
Xu, Y. et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol. Appl. Pharmacol. 259, 395–401 (2012).
Hasegawa, K. et al. Sirt1 protects against oxidative stress–induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. 372, 51–56 (2008).
Hasegawa, K. et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 285, 13045–13056 (2010).
Gilbert, R.E. & Cooper, M.E. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 56, 1627–1637 (1999).
Nedachi, T., Kadotani, A., Ariga, M., Katagiri, H. & Kanzaki, M. Ambient glucose levels qualify the potency of insulin myogenic actions by regulating SIRT1 and FoxO3a in C2C12 myocytes. Am. J. Physiol. Endocrinol. Metab. 294, E668–E678 (2008).
Orimo, M. et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 29, 889–894 (2009).
Gibb, D.M. et al. Renal tubular proteinuria and microalbuminuria in diabetic patients. Arch. Dis. Child. 64, 129–134 (1989).
Vaidya, V.S. et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int. 79, 464–470 (2011).
Bonventre, J.V. Can we target tubular damage to prevent renal function decline in diabetes? Semin. Nephrol. 32, 452–462 (2012).
Ohse, T. et al. De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am. J. Physiol. Renal Physiol. 298, F702–F711 (2010).
Zhang, J. et al. De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am. J. Physiol. Renal Physiol. 302, F571–F580 (2012).
Huby, A.C. et al. Restoration of podocyte structure and improvement of chronic renal disease in transgenic mice overexpressing renin. PLoS ONE 4, e6721 (2009).
Ohse, T. et al. A new function for parietal epithelial cells: a second glomerular barrier. Am. J. Physiol. Renal Physiol. 297, F1566–F1574 (2009).
Rincon-Choles, H. et al. ZO-1 expression and phosphorylation in diabetic nephropathy. Diabetes 55, 894–900 (2006).
Zhang, Y. et al. Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. J. Am. Soc. Nephrol. 17, 1090–1101 (2006).
Dai, C. et al. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 20, 1997–2008 (2009).
Dhawan, P. et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Invest. 115, 1765–1776 (2005).
Leelahavanichkul, A. et al. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 78, 1136–1153 (2010).
Boireau, S. et al. DNA-methylation–dependent alterations of Claudin-4 expression in human bladder carcinoma. Carcinogenesis 28, 246–258 (2007).
Turek-Plewa, J. & Jagodziński, P.P. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell Mol. Biol. Lett. 10, 631–647 (2005).
Fuks, F. DNA methylation and histone modifications: teaming up to silence genes. Curr. Opin. Genet. Dev. 15, 490–495 (2005).
Peng, L. et al. SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol. Cell. Biol. 31, 4720–4734 (2011).
O'Hagan, H.M., Mohammad, H.P. & Baylin, S.B. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genet. 4, e1000155 (2008).
Zhang, Q. et al. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc. Natl. Acad. Sci. USA 104, 829–833 (2007).
Wang, P. et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann. Neurol. 69, 360–374 (2011).
Ramsey, K.M. et al. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell–specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7, 78–88 (2008).
Wang, P. et al. Perivascular adipose tissue–derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc. Res. 81, 370–380 (2009).
Formentini, L., Moroni, F. & Chiarugi, A. Detection and pharmacological modulation of nicotinamide mononucleotide (NMN) in vitro and in vivo. Biochem. Pharmacol. 77, 1612–1620 (2009).
Taha, H.M. et al. Molecular analysis of the interaction of anthrax adenylyl cyclase toxin, edema factor, with 2′(3′)-O-(N-(methyl)anthraniloyl)–substituted purine and pyrimidine nucleotides. Mol. Pharmacol. 75, 693–703 (2009).
Lai, L.W., Moeckel, G.W. & Lien, Y.H. Kidney-targeted liposome-mediated gene transfer in mice. Gene Ther. 4, 426–431 (1997).
Zhang, Z. et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 (2007).
Peng, L. et al. Sirt1 deacetylates the DNA methyltransferase 1 (Dnmt1) protein and alters its activities. Mol. Cell Biol. 31, 4720–4734 (2011).
Zorzano, A., Zorzano, A., Hernández-Alvarez, M.I., Palacín, M. & Mingrone, G. Alterations in the mitochondrial regulatory pathways constituted by the nuclear co-factors PGC-1α or PGC-1β and mitofusin 2 in skeletal muscle in type 2 diabetes. Biochim. Biophys. Acta 1797, 1028–1033 (2010).
Nakahata, Y., Sahar, S., Astarita, G., Kaluzova, M. & Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009).
Clodfelder-Miller, B., Sarno, P.D., Zmijewska, A.A., Song, L. & Jope, R.S. Physiological and pathological changes in glucose regulate brain Akt and glycogen synthase kinase-3. J. Biol. Chem. 280, 39723–39731 (2005).
Leotlela, P.D. et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene 26, 3846–3856 (2007).
Moeller, M.J., Sanden, S.K., Soofi, A., Wiggins, R.C. & Holzman, L.B. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35, 39–42 (2003).
Sung, S.H. et al. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J. Am. Soc. Nephrol. 17, 3093–3104 (2006).
Cohen, C.D. et al. Laser microdissection and gene expression analysis on formaldehyde-fixed archival tissue. Kidney Int. 61, 125–132 (2002).
Kohda, Y. et al. Analysis of segmental renal gene expression by laser capture microdissection. Kidney Int. 57, 321–331 (2000).
Asanuma, K., Campbell, K.N., Kim, K., Faul, C. & Mundel, P. Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes. Proc. Natl. Acad. Sci. USA 104, 10134–10139 (2007).
Terasawa, K. et al. Epigenetic inactivation of TMS1/ASC in ovarian cancer. Clin. Cancer Res. 10, 2000–2006 (2004).
Acknowledgements
We thank P. Mundel (Division of Nephrology, Massachusetts General Hospital and Harvard Medical School) and K. Asanuma (Division of Nephrology, Department of Internal Medicine, Juntendo University Faculty of Medicine) for providing cultured podocytes. We also thank S.J. Shankland and C. Campbell (Division of Nephrology, University of Washington) for providing culture PECs. This work was supported by the Scientific Research Fund of the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant 22790800).
Author information
Authors and Affiliations
Contributions
K. Hasegawa, S.W., L.G. and H.I. designed the experiments and the study. K. Hasegawa, P.S., Y.S. and E.K. collected data or performed experiments for the study. K. Hasegawa, S.W., P.S., H.M., K.F., K. Hosoya, M.K., Y.K., T.K., H.T., K. Hayashi, L.G. and H.I. analyzed the data and contributed to writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Figure Legends, Supplementary Results and Supplementary Tables 1–6 (PDF 7721 kb)
Rights and permissions
About this article
Cite this article
Hasegawa, K., Wakino, S., Simic, P. et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 19, 1496–1504 (2013). https://doi.org/10.1038/nm.3363
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3363
This article is cited by
-
Sirtuins in kidney diseases: potential mechanism and therapeutic targets
Cell Communication and Signaling (2024)
-
Sirtuins in kidney health and disease
Nature Reviews Nephrology (2024)
-
Adverse effects of acute tubular injury on the glomerulus: contributing factors and mechanisms
Pediatric Nephrology (2024)
-
Protective effect of Cordyceps sinensis against diabetic kidney disease through promoting proliferation and inhibiting apoptosis of renal proximal tubular cells
BMC Complementary Medicine and Therapies (2023)
-
Cellular crosstalk of mesangial cells and tubular epithelial cells in diabetic kidney disease
Cell Communication and Signaling (2023)