Abstract
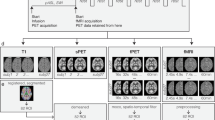
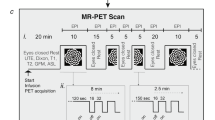
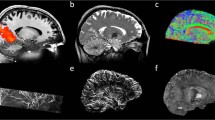
Combined positron emission tomography (PET) and magnetic resonance imaging (MRI) is a new tool to study functional processes in the brain. Here we study brain function in response to a barrel-field stimulus simultaneously using PET, which traces changes in glucose metabolism on a slow time scale, and functional MRI (fMRI), which assesses fast vascular and oxygenation changes during activation. We found spatial and quantitative discrepancies between the PET and the fMRI activation data. The functional connectivity of the rat brain was assessed by both modalities: the fMRI approach determined a total of nine known neural networks, whereas the PET method identified seven glucose metabolism–related networks. These results demonstrate the feasibility of combined PET-MRI for the simultaneous study of the brain at activation and rest, revealing comprehensive and complementary information to further decode brain function and brain networks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ogawa, S., Lee, T.M., Kay, A.R. & Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 87, 9868–9872 (1990).
Phelps, M.E. & Mazziotta, J.C. Positron emission tomography: human brain function and biochemistry. Science 228, 799–809 (1985).
Buxton, R.B. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front. Neuroenergetics 2, 8 (2010).
Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Biswal, B.B., Van Kylen, J. & Hyde, J.S. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10, 165–170 (1997).
Zhang, D. & Raichle, M.E. Disease and the brain's dark energy. Nat. Rev. Neurol. 6, 15–28 (2010).
Shih, Y.Y., Wey, H.Y., De La Garza, B.H. & Duong, T.Q. Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. J. Cereb. Blood Flow Metab. 31, 832–841 (2011).
Kinahan, P.E. & Noll, D.C. A direct comparison between whole-brain PET and BOLD fMRI measurements of single-subject activation response. Neuroimage 9, 430–438 (1999).
Judenhofer, M.S. et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med. 14, 459–465 (2008).
Schlemmer, H.P. et al. Simultaneous MR/PET imaging of the human brain: feasibility study. Radiology 248, 1028–1035 (2008).
Hjornevik, T. et al. Metabolic plasticity in the supraspinal pain modulating circuitry after noxious stimulus-induced spinal cord LTP. Pain 140, 456–464 (2008).
Magistretti, P.J. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 209, 2304–2311 (2006).
Wehrl, H.F. et al. Assessment of MR compatibility of a PET insert developed for simultaneous multiparametric PET/MR imaging on an animal system operating at 7 T. Magn. Reson. Med. 65, 269–279 (2011).
Shimoji, K. et al. Measurement of cerebral glucose metabolic rates in the anesthetized rat by dynamic scanning with 18F-FDG, the ATLAS small animal PET scanner, and arterial blood sampling. J. Nucl. Med. 45, 665–672 (2004).
Jonckers, E., Van Audekerke, J., De Visscher, G., Van der Linden, A. & Verhoye, M. Functional connectivity fMRI of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS ONE 6, e18876 (2011).
Paxinos, G. & Watson, C. The Rat Brain in Sterotaxic Coordinates (Academic Press, San Diego, 1998).
Kennerley, A.J., Mayhew, J.E., Redgrave, P. & Berwick, J. Vascular origins of BOLD and CBV fMRI signals: statistical mapping and histological sections compared. Open Neuroimag. J. 4, 1–8 (2010).
Sanganahalli, B.G., Herman, P. & Hyder, F. Frequency-dependent tactile responses in rat brain measured by functional MRI. NMR Biomed. 21, 410–416 (2008).
Kornblum, H.I. et al. In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET). Nat. Biotechnol. 18, 655–660 (2000).
Ravasi, L. et al. Use of [18F]fluorodeoxyglucose and the ATLAS small animal PET scanner to examine cerebral functional activation by whisker stimulation in unanesthetized rats. Nucl. Med. Commun. 32, 336–342 (2011).
Koyama, Y., Koyama, T., Kroncke, A.P. & Coghill, R.C. Effects of stimulus duration on heat induced pain: the relationship between real-time and post-stimulus pain ratings. Pain 107, 256–266 (2004).
Tran, T.D., Wang, H., Tandon, A., Hernandez-Garcia, L. & Casey, K.L. Temporal summation of heat pain in humans: evidence supporting thalamocortical modulation. Pain 150, 93–102 (2010).
Yu, X. et al. Direct imaging of macrovascular and microvascular contributions to BOLD fMRI in layers IV–V of the rat whisker-barrel cortex. Neuroimage 59, 1451–1460 (2012).
Backes, H. et al. Whiskers area as extracerebral reference tissue for quantification of rat brain metabolism using 18F-FDG PET: application to focal cerebral ischemia. J. Nucl. Med. 52, 1252–1260 (2011).
Bosshard, S.C. et al. Assessment of brain responses to innocuous and noxious electrical forepaw stimulation in mice using BOLD fMRI. Pain 151, 655–663 (2010).
Fox, P.T., Raichle, M.E., Mintun, M.A. & Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464 (1988).
Kim, S.G. & Ogawa, S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 32, 1188–1206 (2012).
Hutchison, R.M., Mirsattari, S.M., Jones, C.K., Gati, J.S. & Leung, L.S. Functional networks in the anesthetized rat brain revealed by independent component analysis of resting-state FMRI. J. Neurophysiol. 103, 3398–3406 (2010).
Vaishnavi, S.N. et al. Regional aerobic glycolysis in the human brain. Proc. Natl. Acad. Sci. USA 107, 17757–17762 (2010).
Fox, M.D. & Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007).
Acknowledgements
We thank F. Cay and M. Koenig for excellent technical support during this project. This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft PI 771/1-1, PI 771/5-1), the Wilhelm Schuler-Foundation and the Werner Siemens-Foundation.
Author information
Authors and Affiliations
Contributions
H.F.W. and B.J.P. designed the study, evaluated and analyzed data and prepared the manuscript, H.F.W., M.H., K.L., C.-C.L., I.B. and B.J.P. developed the PET insert, G.R. produced the radiotracer, H.F.W. and M.H. made PET-MRI measurements, H.F.W., P.M. and F.S. developed the MRI sequence, and all authors read and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.J.P. receives grant and research support from AstraZeneca, Bayer Healthcare, Boehringer-Ingelheim, Bruker, Oncodesign, Merck, Siemens and the Werner Siemens-Foundation. K.L. is an employee of Bruker.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, Supplementary Tables 1 and 2, Supplementary Results, Supplementary Discussion and Supplementary Methods (PDF 5899 kb)
Rights and permissions
About this article
Cite this article
Wehrl, H., Hossain, M., Lankes, K. et al. Simultaneous PET-MRI reveals brain function in activated and resting state on metabolic, hemodynamic and multiple temporal scales. Nat Med 19, 1184–1189 (2013). https://doi.org/10.1038/nm.3290
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3290
This article is cited by
-
Neuroimaging in psychedelic drug development: past, present, and future
Molecular Psychiatry (2023)
-
Machine learning identifies multi-parametric functional PET/MR imaging cluster to predict radiation resistance in preclinical head and neck cancer models
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Exercise alters cortico-basal ganglia network metabolic connectivity: a mesoscopic level analysis informed by anatomic parcellation defined in the mouse brain connectome
Brain Structure and Function (2023)
-
Precision targeting in prediction for rTMS clinical outcome in depression: what about sgACC lateralization, metabolic connectivity, and the potential role of the cerebellum?
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Depletion and activation of microglia impact metabolic connectivity of the mouse brain
Journal of Neuroinflammation (2023)