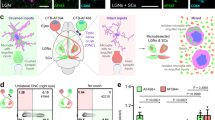
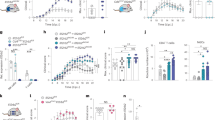
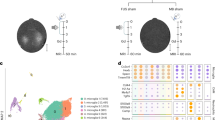
Abstract
Multiple sclerosis is an autoimmune disease of the central nervous system (CNS) that is initiated when self-reactive T cells enter the brain and become locally activated after encountering their specific nervous antigens. When and where the disease-relevant antigen encounters occur is unclear. Here we combined fluorescently labeled nuclear factor of activated T cells (NFAT) with histone protein H2B to create a broadly applicable molecular sensor for intravital imaging of T cell activation. In experimental autoimmune encephalomyelitis, an animal model for multiple sclerosis, we report that effector T cells entering the CNS become activated after short contacts with leptomeningeal phagocytes. During established disease, the activation process is extended to the depth of the CNS parenchyma, where the cells form contacts with microglia and recruited phagocytes. We show that it is the activation processes during the preclinical phase rather than during established disease that are essential for the intensity and duration of the disease bout.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Chastain, E.M., Duncan, D.S., Rodgers, J.M. & Miller, S.D. The role of antigen presenting cells in multiple sclerosis. Biochim. Biophys. Acta 1812, 265–274 (2011).
Shaw, J.P. et al. Identification of a putative regulator of early T cell activation genes. Science 241, 202–205 (1988).
Odoardi, F. et al. T cells become licensed in the lung to enter the central nervous system. Nature 488, 675–679 (2012).
Bartholomäus, I. et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009).
McMenamin, P.G. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol. 405, 553–562 (1999).
Moolten, F.L. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 46, 5276–5281 (1986).
Croxford, A.L. & Buch, T. Cytokine reporter mice in immunological research: perspectives and lessons learned. Immunology 132, 1–8 (2011).
Magness, S.T. et al. In vivo pattern of lipopolysaccharide and anti-CD3–induced NF-κB activation using a novel gene-targeted enhanced GFP reporter gene mouse. J. Immunol. 173, 1561–1570 (2004).
Saparov, A. et al. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity 11, 271–280 (1999).
Friedman, R.S., Beemiller, P., Sorensen, C.M., Jacobelli, J. & Krummel, M.F. Real-time analysis of T cell receptors in naive cells in vitro and in vivo reveals flexibility in synapse and signaling dynamics. J. Exp. Med. 207, 2733–2749 (2010).
Azar, G.A., Lemaitre, F., Robey, E.A. & Bousso, P. Subcellular dynamics of T cell immunological synapses and kinapses in lymph nodes. Proc. Natl. Acad. Sci. USA 107, 3675–3680 (2010).
Thauland, T.J. & Parker, D.C. Diversity in immunological synapse structure. Immunology 131, 466–472 (2010).
Gunzer, M. et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity 13, 323–332 (2000).
Schubert, D.A. et al. Self-reactive human CD4 T cell clones form unusual immunological synapses. J. Exp. Med. 209, 335–352 (2012).
Skokos, D. et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat. Immunol. 8, 835–844 (2007).
Revy, P., Sospedra, M., Barbour, B. & Trautmann, A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2, 925–931 (2001).
Kondo, T. et al. Dendritic cells signal T cells in the absence of exogenous antigen. Nat. Immunol. 2, 932–938 (2001).
Masuda, E.S., Imamura, R., Amasaki, Y., Arai, K. & Arai, N. Signalling into the T-cell nucleus: NFAT regulation. Cell Signal. 10, 599–611 (1998).
Loh, C., Carew, J.A., Kim, J., Hogan, P.G. & Rao, A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol. Cell Biol. 16, 3945–3954 (1996).
Kanda, T., Sullivan, K.F. & Wahl, G.M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8, 377–385 (1998).
Foudi, A. et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 27, 84–90 (2009).
Shibasaki, F., Price, E.R., Milan, D. & McKeon, F. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature 382, 370–373 (1996).
Tomida, T., Hirose, K., Takizawa, A., Shibasaki, F. & Lino, M. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. EMBO J. 22, 3825–3832 (2003).
Lassmann, H. & Wisniewski, H.M. Chronic relapsing EAE. Time course of neurological symptoms and pathology. Acta Neuropathol. 43, 35–42 (1978).
Kawakami, N. et al. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 199, 185–197 (2004).
Kivisäkk, P. et al. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann. Neurol. 65, 457–469 (2009).
Odoardi, F. et al. Instant effect of soluble antigen on effector T cells in peripheral immune organs during immunotherapy of autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 104, 920–925 (2007).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Hugues, S. et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat. Immunol. 5, 1235–1242 (2004).
Cahalan, M.D. & Parker, I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu. Rev. Immunol. 26, 585–626 (2008).
Kim, J.V., Kang, S.S., Dustin, M.L. & McGavern, D.B. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195 (2009).
Kang, S.S. et al. Migration of cytotoxic lymphocytes in cell cycle permits local MHC I–dependent control of division at sites of viral infection. J. Exp. Med. 208, 747–759 (2011).
Heppner, F.L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 (2005).
Hirasawa, T. et al. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J. Neurosci. Res. 81, 357–362 (2005).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328–334 (2005).
McMahon, E.J., Bailey, S.L., Castenada, C.V., Waldner, H. & Miller, S.D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Acknowledgements
We thank N. Meyer, S. Hamann, A. Stas and M. Weig for excellent technical assistance. We thank R. Tsien (Department of Chemistry and Biochemistry, University of California at San Diego, La Jolla) for providing mCherry complementary DNA (cDNA). We also thank C. Ludwig for text editing and M.B. Graeber and F. Lühder for critical reading of the manuscript. D.L., F.O. and A.F. were supported by the Deutsche Forschungsgemeinschaft (FOR 1336, FL 377/2-7; TRR-SFB43 B10 and B11), the Bundesministerium für Bildung und Forschung ('UNDERSTAND MS') and the Hertie Foundation (grant 1.01.1/11/004). The work of H.M.R.'s lab was supported by the Deutsche Forschungsgemeinschaft (RE 1631/10-1; SFB-TRR43 B13).
Author information
Authors and Affiliations
Contributions
D.L. designed and cloned the sensors, established and characterized T cell lines and performed APC preparation, video microscopy analyses and EAE experiments and treatments. F.O. performed quantitative PCR analyses and together with C.S. generated the intravital imaging data. H.K. and D.L. performed histological preparations, staining, fluorescent microscopy and data analyses. J.v.d.B. and H.M.R. generated AsRed-transgenic Lewis rats. M.H. contributed to 2-PM analyses of acute spinal cord slices. A.K. contributed to cloning of constructs and molecular analyses of T cells. M.N. contributed to establishing the HSV-TK suicide system. A.F. together with D.L. and F.O. designed the study, coordinated the experimental work and wrote the manuscript with input from the coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Methods (PDF 2908 kb)
Supplementary Video 1
NFAT translocation in T cells upon interaction with DCs in vitro. Dendritic cells were derived from bone marrow of AsRed-transgenic rats. After stimulation with recombinant rat IFN-γ, DCs were loaded with antigen and co-cultured with resting TMBP-NFAT/YFP-Cherry/H2B cells in a conditioned glassbottom imaging chamber. Representative time lapse epifluorescence recording is shown. Green, NFAT-YFP; red, AsRed (DC) and mCherry-H2B (T cells). White and yellow arrows, T cells before and after nuclear NFAT translocation, respectively. Time interval, 1 min. Scale bar, 10 μm. (MOV 428 kb)
Supplementary Video 2
Nuclear NFAT translocation under antigenic challenge in popliteal LNs. Animals received s.c. injection of resting TMBP-NFAT/YFP-Cherry/H2B cells. 20 h later, they were immunized s.c. with either PBS (control, left) or MBP (immunized, right). 2-PM recordings of the draining popliteal lymph nodes were performed 4 h thereafter. Progressive trajectories of individual motile T cells are shown. White and yellow lines depict tracks of T cells with cytosolic (not translocated) or nuclear (translocated) NFAT, respectively. Scale bar, 50 μm. Time intervals, 41.2 s (left), 43.6 s (right). Representative videos of at least 3 independent experiments are shown. (MOV 2027 kb)
Supplementary Video 3
Nuclear NFAT translocations in TMBP-NFAT/YFP-Cherry/H2B cells do not occur within CNS blood vessels. 2-PM recordings of TMBP-NFAT/YFP-Cherry/H2B cells within the leptomeningeal vessel during the preclinical phase are shown. Progressive trajectories of intraluminal (left) and extravasated TMBP-NFAT/YFP-Cherry/H2B cells (right) are depicted. Insets: magnified regions with their origins indicated by white frames. White and yellow lines depict tracks of TMBP-NFAT/YFP-Cherry/H2B cells with cytosolic (not translocated) or nuclear NFAT (translocated), respectively. Blood vessels and meningeal phagocytes were labeled by i.v. or i.t. injection of TR-dextran respectively. Scale bar, 50 μm. Time interval, 33.9 s. Representative video of at least 3 independent experiments is shown. (MOV 635 kb)
Supplementary Video 4
De novo nuclear translocation of NFAT biosensor in a TMBP-NFAT/YFP-H2B/Cherry cell in leptomeninges of the spinal cord. 2-PM data of the leptomeninges in the preclinincal phase of EAE are shown. Blood vessels and meningeal phagocytes were labeled as described for Supplementary Video 3; yellow line (dotted): perivascular meningeal phagocyte; arrows indicate T cell position and status before (white) and after nuclear translocation of NFAT biosensor (yellow). Scale bar, 50 μm. Time interval, 35.5 s. Representative video of at least 3 independent experiments is shown. (MOV 635 kb)
Supplementary Video 5
Induction of nuclear NFAT translocation in TMBP-NFAT/YFP-Cherry/H2B cells after antigen challenge. 2-PM videos were recorded during the acute phase of EAE. During the imaging i.v. injection of MBP was performed. Representative spots before (left) and 2 h after soluble MBP injection (right) are shown. Post-production videos are depicted. Lines: trajectories of TMBP-NFAT/YFP-Cherry/H2B cells with either cytoplasmic (white) or nuclear NFAT (yellow). Circles: stationary TMBP-NFAT/YFP-Cherry/H2B cells with cytoplasmic (white) or nuclear NFAT biosensor (yellow). Red: meningeal phagocytes. Scale bar, 50 μm; Time interval, 47.6 s. Representative videos of at least 3 independent experiments are shown. (MOV 1532 kb)
Supplementary Video 6
Visualization of mitosis in a TMBP-NFAT/YFP-Cherry/H2B cell within the CNS. A TMBP-NFAT/YFP-Cherry/H2B cell is shown undergoing mitosis in the CNS during the clinical phase of EAE. Inset: magnified region with its origin indicated by white frames. White arrows: position of an individual T cells; meningeal phagocytes were labeled TR-dextran (red). Scale bar, 50 μm. Time interval, 47.8 s. (MOV 918 kb)
Supplementary Video 7
Motility of TMBP-NFAT/YFP-Cherry/H2B cells within the leptomeninges during the acute phase of EAE. TMBP-NFAT/YFP-Cherry/H2B cells were recorded by 2-PM during the acute phase of the disease. Left: trajectories of motile T cells with nuclear or cytoplasmic NFAT biosensor (yellow and white lines, respectively). Right: stationary T cells with nuclear or cytoplasmic NFAT sensor (yellow and white circles, respectively). Scale bar, 50 μm. Time interval, 35.9 s. A representative video of at least 3 independent experiments is shown. (MOV 900 kb)
Supplementary Video 8
TOVA-NFAT/YFP-Cherry/H2B cells that cannot recognize CNS antigens are not activated in the leptomeninges. 2-PM movies of TOVA-NFAT/YFP-Cherry/H2B cells (co-injected with unlabeled TMBP cells) during the acute phase of the disease are shown. Trajectories of motile (white lines, left) or stationary (white circles, right) T cells with cytoplasmic NFAT sensor are depicted. Scale bar, 50 μm. Time interval, 38.9 s. A representative video of at least 3 independent experiments is shown. (MOV 253 kb)
Supplementary Video 9
Activated TMBP-NFAT/YFP lymphocyte in contact with a resident Iba1+ cell. Maximum intensity projection (left) and animated 3D-reconstruction (right) of a confocal image stack are shown. Images were acquired at the meningeal/subpial white matter interface of the lumbal spinal cord of an animal with preclinical EAE. Tissue section was stained with anti-Iba1 antibody (red) and DAPI. Scale bar, 10 μm. (MOV 253 kb)
Supplementary Video 10
De novo nuclear translocation of NFAT biosensor in CNS parenchyma. 2-PM data of acute CNS slices at the peak of clinical EAE are depicted. Arrows indicate T cell position and activation status (white, before nuclear translocation of NFAT biosensor; yellow, after translocation of NFAT). Scale bar, 20 μm. Time interval, 32 s. A representative video of at least 3 independent experiments is shown. (MOV 307 kb)
Rights and permissions
About this article
Cite this article
Lodygin, D., Odoardi, F., Schläger, C. et al. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat Med 19, 784–790 (2013). https://doi.org/10.1038/nm.3182
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3182
This article is cited by
-
Neoantigen-targeted TCR-engineered T cell immunotherapy: current advances and challenges
Biomarker Research (2023)
-
Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis
Nature Reviews Neurology (2023)
-
The lung microbiome regulates brain autoimmunity
Nature (2022)
-
β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration
Nature (2019)
-
Coupled feedback regulation of nuclear factor of activated T-cells (NFAT) modulates activation-induced cell death of T cells
Scientific Reports (2019)