Abstract
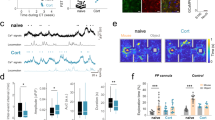
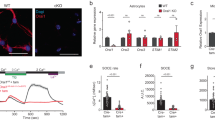
Major depressive disorder (MDD) is a cause of disability that affects approximately 16% of the world's population1; however, little is known regarding the underlying biology of this disorder. Animal studies, postmortem brain analyses and imaging studies of patients with depression have implicated glial dysfunction in MDD pathophysiology2,3,4,5,6,7. However, the molecular mechanisms through which astrocytes modulate depressive behaviors are largely uncharacterized. Here, we identified ATP as a key factor involved in astrocytic modulation of depressive-like behavior in adult mice. We observed low ATP abundance in the brains of mice that were susceptible to chronic social defeat. Furthermore, we found that the administration of ATP induced a rapid antidepressant-like effect in these mice. Both a lack of inositol 1,4,5-trisphosphate receptor type 2 and transgenic blockage of vesicular gliotransmission induced deficiencies in astrocytic ATP release, causing depressive-like behaviors that could be rescued via the administration of ATP. Using transgenic mice that express a Gq G protein–coupled receptor only in astrocytes to enable selective activation of astrocytic Ca2+ signaling, we found that stimulating endogenous ATP release from astrocytes induced antidepressant-like effects in mouse models of depression. Moreover, we found that P2X2 receptors in the medial prefrontal cortex mediated the antidepressant-like effects of ATP. These results highlight astrocytic ATP release as a biological mechanism of MDD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Kessler, R.C. et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). J. Am. Med. Assoc. 289, 3095–3105 (2003).
Ongür, D., Drevets, W.C. & Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 95, 13290–13295 (1998).
Rajkowska, G. et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 45, 1085–1098 (1999).
Cotter, D., Mackay, D., Landau, S., Kerwin, R. & Everall, I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 58, 545–553 (2001).
Sheline, Y.I., Gado, M.H. & Kraemer, H.C. Untreated depression and hippocampal volume loss. Am. J. Psychiatry 160, 1516–1518 (2003).
Banasr, M. & Duman, R.S. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry 64, 863–870 (2008).
Banasr, M. et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 15, 501–511 (2010).
Berton, O. et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868 (2006).
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007).
Stockmeier, C.A. et al. Cellular changes in the postmortem hippocampus in major depression. Biol. Psychiatry 56, 640–650 (2004).
Zhang, Q. et al. Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 279, 12724–12733 (2004).
Zou, C.J., Onaka, T.O. & Yagi, K. Effects of suramin on neuroendocrine and behavioral responses to conditioned fear stimuli. Neuroreport 9, 997–999 (1998).
Monleon, S. et al. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl.) 117, 453–457 (1995).
Surget, A. et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry 64, 293–301 (2008).
Marpegan, L. et al. Circadian regulation of ATP release in astrocytes. J. Neurosci. 31, 8342–8350 (2011).
Sharp, A.H. et al. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J. Comp. Neurol. 406, 207–220 (1999).
Holtzclaw, L.A., Pandhit, S., Bare, D.J., Mignery, G.A. & Russell, J.T. Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia 39, 69–84 (2002).
Hertle, D.N. & Yeckel, M.F. Distribution of inositol 1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience 150, 625–638 (2007).
Zhang, Z. et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 (2007).
Petravicz, J., Fiacco, T.A. & McCarthy, K.D. Loss of IP3 receptor–dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28, 4967–4973 (2008).
Agulhon, C., Fiacco, T.A. & McCarthy, K.D. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254 (2010).
Pascual, O. et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116 (2005).
Dong, X., Han, S., Zylka, M.J., Simon, M.I. & Anderson, D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106, 619–632 (2001).
Fiacco, T.A. et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54, 611–626 (2007).
Jacques-Silva, M.C. et al. ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc. Natl. Acad. Sci. USA 107, 6465–6470 (2010).
Khakh, B.S. & North, R.A. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76, 51–69 (2012).
Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 (2007).
Gever, J.R. et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br. J. Pharmacol. 160, 1387–1398 (2010).
Xiong, K. et al. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J. Neurophysiol. 81, 2088–2094 (1999).
Klempan, T.A. et al. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol. Psychiatry 14, 175–189 (2009).
Li, X., Zima, A.V., Sheikh, F., Blatter, L.A. & Chen, J. Endothelin-1–induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol 1,4,5-trisphosphate (IP3) receptor type 2–deficient mice. Circ. Res. 96, 1274–1281 (2005).
Halassa, M.M. et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219 (2009).
Cotrina, M.L. et al. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 95, 15735–15740 (1998).
Alonso, R. et al. Blockade of CRF (1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol. Psychiatry 9, 278–286, 224 (2004).
Roybal, K. et al. Mania-like behavior induced by disruption of CLOCK. Proc. Natl. Acad. Sci. USA 104, 6406–6411 (2007).
Monleon, S. et al. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl.) 117, 453–457 (1995).
Acknowledgements
We thank J. Chen (University of California, San Diego) for providing Itpr2+/− mice and P.G. Haydon (Tufts University School of Medicine, Boston) for providing GFAP-tTA and tetO.SNARE mouse lines. We thank L. Mei for his insightful suggestions. This work was partly supported by the National Natural Science Foundation of China (grants 81171276, 8103002 and U1201225), the Key Project of Guangdong Province (grants 9351051501000003 and CXZB1018), the Guangzhou Science and Technology Project (grant 7411802013939), the Major State Basic Research Program of China (grant 2012CB518203), the Program for Changjiang Scholars and Innovative Research Team in University (grant IRT1142) and the Project supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2011).
Author information
Authors and Affiliations
Contributions
X.-H.Z. and T.-M.G. designed the research. X.C., L.-P.L., Q. Wang, J.Z., W.-C.X., Y.-B.G., X.-W.L., Y.-Y.F., Y.-N.Z. and H.-C.Y. conducted the behavioral tests and analysis. X.C., L.-P. L., Q. Wu, M.Z. and J.-H.L. performed the ELISA and ATP measurements. X.C. and L.-R.S. contributed to the HPLC analysis. X.C. performed the immunofluorescence, calcium imaging, osmotic minipump implantation and stereotaxic microinjection. L.-P.L., X.C. and S.-J.L. performed the cell culture. Q. Wu and H.-H.H. performed the western blotting. X.-H.Z., T.-M.G. and X.C. analyzed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18 and Supplementary Methods (PDF 2792 kb)
Rights and permissions
About this article
Cite this article
Cao, X., Li, LP., Wang, Q. et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19, 773–777 (2013). https://doi.org/10.1038/nm.3162
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3162
This article is cited by
-
Puerarin attenuates valproate-induced features of ASD in male mice via regulating Slc7a11-dependent ferroptosis
Neuropsychopharmacology (2024)
-
Cortical astrocyte N-methyl-D-aspartate receptors influence whisker barrel activity and sensory discrimination in mice
Nature Communications (2024)
-
Dorsal hippocampal astrocytes mediate the development of heroin withdrawal-enhanced fear learning
Psychopharmacology (2024)
-
The mitochondrial Ahi1/GR participates the regulation on mtDNA copy numbers and brain ATP levels and modulates depressive behaviors in mice
Cell Communication and Signaling (2023)
-
LHPP, a risk factor for major depressive disorder, regulates stress-induced depression-like behaviors through its histidine phosphatase activity
Molecular Psychiatry (2023)