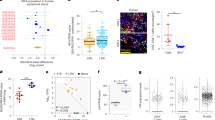
Abstract
The majority of HIV-infected individuals fail to produce protective antibodies and have diminished responses to new immunizations1,2,3. We report here that even though there is an expansion of follicular helper T (TFH) cells in HIV-infected individuals, the cells are unable to provide adequate B cell help. We found a higher frequency of programmed cell death ligand 1 (PD-L1)+ germinal center B cells from lymph nodes of HIV-infected individuals suggesting a potential role for PD-1–PD-L1 interaction in regulating TFH cell function. In fact, we show that engagement of PD-1 on TFH cells leads to a reduction in cell proliferation, activation, inducible T-cell co-stimulator (ICOS) expression and interleukin-21 (IL-21) cytokine secretion. Blocking PD-1 signaling enhances HIV-specific immunoglobulin production in vitro. We further show that at least part of this defect involves IL-21, as addition of this cytokine rescues antibody responses and plasma cell generation in vitro. Our results suggest that deregulation of TFH cell–mediated B cell help diminishes B cell responses during HIV infection and may be related to PD-1 triggering on TFH cells. These results demonstrate a role for TFH cell impairment in HIV pathogenesis and suggest that enhancing their function could have a major impact on the outcome and control of HIV infection, preventing future infections and improving immune responses to vaccinations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
De Milito, A. et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103, 2180–2186 (2004).
Titanji, K. et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 108, 1580–1587 (2006).
Hart, M. et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J. Immunol. 178, 8212–8220 (2007).
Moir, S. & Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 9, 235–245 (2009).
van Grevenynghe, J. et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J. Clin. Invest. 121, 3877–3888 (2011).
De Milito, A., Morch, C., Sonnerborg, A. & Chiodi, F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS 15, 957–964 (2001).
Scheid, J.F. et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458, 636–640 (2009).
Walker, L.M. et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470 (2011).
Wu, X. et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329, 856–861 (2010).
Fahey, L.M. et al. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J. Exp. Med. 208, 987–999 (2011).
Harker, J.A., Lewis, G.M., Mack, L. & Zuniga, E.I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011).
Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 (2011).
Haase, A.T. et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274, 985–989 (1996).
Keele, B.F. et al. Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J. Virol. 82, 5548–5561 (2008).
Lindqvist, M. et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 122, 3271–3280 (2012).
Hong, J.J., Amancha, P.K., Rogers, K., Ansari, A.A. & Villinger, F. Spatial alterations between CD4+ T follicular helper, B, and CD8+ T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J. Immunol. 188, 3247–3256 (2012).
Petrovas, C. et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 122, 3281–3294 (2012).
Popovic, M. et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 102, 14807–14812 (2005).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 (2008).
Malaspina, A. et al. CpG oligonucleotides enhance proliferative and effector responses of B cells in HIV-infected individuals. J. Immunol. 181, 1199–1206 (2008).
Karnowski, A. et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J. Exp. Med. 209, 2049–2064 (2012).
Choi, Y.S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Konforte, D., Simard, N. & Paige, C.J. IL-21: an executor of B cell fate. J. Immunol. 182, 1781–1787 (2009).
Sage, P.T., Francisco, L.M., Carman, C.V. & Sharpe, A.H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2012).
Linterman, M.A. et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17, 975–982 (2011).
Chung, Y. et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17, 983–988 (2011).
Wollenberg, I. et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187, 4553–4560 (2011).
Hams, E. et al. Blockade of B7–H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J. Immunol. 186, 5648–5655 (2011).
Bauquet, A.T. et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10, 167–175 (2009).
Kroenke, M.A. et al. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 188, 3734–3744 (2012).
Kim, J.I., Ho, I.C., Grusby, M.J. & Glimcher, L.H. The transcription factor c-Maf controls the production of interleukin-4 but not other TH2 cytokines. Immunity 10, 745–751 (1999).
Iannello, A. et al. Decreased levels of circulating IL-21 in HIV-infected AIDS patients: correlation with CD4+ T-cell counts. Viral Immunol. 21, 385–388 (2008).
Wei, X. et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003).
Francisco, L.M., Sage, P.T. & Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 236, 219–242 (2010).
Butler, N.S. et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat. Immunol. 13, 188–195 (2012).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458, 206–210 (2009).
Bekker, V. et al. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics 118, e315–e322 (2006).
Mehta, N. et al. Impaired generation of hepatitis B virus–specific memory B cells in HIV infected individuals following vaccination. Vaccine 28, 3672–3678 (2010).
Klatt, N.R. et al. SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood 118, 5803–5812 (2011).
Schacker, T.W. et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 110, 1133–1139 (2002).
Acknowledgements
We thank W. Ryzner, R. Bordi and D. Brown for sample collection. We also thank K. Kusser and Y. Shi from the Flow Cytometry Core at Vaccine & Gene Therapy Institute Florida for their help with sorting all the samples. We are grateful to R. Debernardo (Case Western Reserve University and University Hospitals Case Medical Center) for obtaining the HIV-uninfected lymph nodes and to B. Rodriguez for his help with the infected lymph node samples. This study was supported in part by US National Institutes of Health (NIH) contract HHSN272201100017C, NIH-1P01 AI080192, U19AI082630, 2P01 AI056299 and R01 AI096966. This work was also supported by the Case Western Reserve University Center for AIDS Research (AI 36219) and by the Cleveland Immunopathogenesis Consortium (AI 76174).
Author information
Authors and Affiliations
Contributions
R.A.C. designed and performed experiments, analyzed data and wrote the manuscript. J.C.M. helped perform the phenotyping of TFH and lymph node cell populations. A.-L.S. and M.P. helped provide preliminary data and performed the Luminex assays. J.v.G. helped in the coculture assays and ELISAs. T.M. assisted in the tonsil processing. E.C. and A.M. provided tissue sections for immunohistochemistry; G.J.F. provided valuable reagents and intellectual input. J.M.J. provided lymph node samples and discussion; A.D.B. performed surgeries on HIV infected lymph nodes; G.A.-T. Jr. provided intellectual input and valuable tonsil samples. S.C. assisted in experimental design, discussion and analysis of data. J.D.E. performed immunohistochemistry. G.P. and M.M.L. provided valuable discussion, designed experiments, provided conceptual advice and edited the manuscript. E.K.H. conceived of and directed the research, analyzed data and helped write the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Table 1 (PDF 819 kb)
Rights and permissions
About this article
Cite this article
Cubas, R., Mudd, J., Savoye, AL. et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19, 494–499 (2013). https://doi.org/10.1038/nm.3109
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3109
This article is cited by
-
The immune response to SARS-CoV-2 in people with HIV
Cellular & Molecular Immunology (2023)
-
CD8 lymphocytes mitigate HIV-1 persistence in lymph node follicular helper T cells during hyperacute-treated infection
Nature Communications (2022)
-
Immunopathogenesis in HIV-associated pediatric tuberculosis
Pediatric Research (2022)
-
A follicular regulatory Innate Lymphoid Cell population impairs interactions between germinal center Tfh and B cells
Communications Biology (2021)
-
Dynamic regulation of TFH selection during the germinal centre reaction
Nature (2021)