Abstract
Oncolytic viruses and active immunotherapeutics have complementary mechanisms of action (MOA) that are both self amplifying in tumors, yet the impact of dose on subject outcome is unclear. JX-594 (Pexa-Vec) is an oncolytic and immunotherapeutic vaccinia virus. To determine the optimal JX-594 dose in subjects with advanced hepatocellular carcinoma (HCC), we conducted a randomized phase 2 dose-finding trial (n = 30). Radiologists infused low- or high-dose JX-594 into liver tumors (days 1, 15 and 29); infusions resulted in acute detectable intravascular JX-594 genomes. Objective intrahepatic Modified Response Evaluation Criteria in Solid Tumors (mRECIST) (15%) and Choi (62%) response rates and intrahepatic disease control (50%) were equivalent in injected and distant noninjected tumors at both doses. JX-594 replication and granulocyte-macrophage colony-stimulating factor (GM-CSF) expression preceded the induction of anticancer immunity. In contrast to tumor response rate and immune endpoints, subject survival duration was significantly related to dose (median survival of 14.1 months compared to 6.7 months on the high and low dose, respectively; hazard ratio 0.39; P = 0.020). JX-594 demonstrated oncolytic and immunotherapy MOA, tumor responses and dose-related survival in individuals with HCC.
Similar content being viewed by others
Main
Despite advances in cancer treatment over the past 30 years with chemotherapy and biologics, the majority of solid tumors remain incurable once they are metastatic. Truly new agents with multiple complementary MOA are required to move beyond the modest benefits achieved so far. Extensive study in the field of active immunotherapy has recently culminated in regulatory approvals of sipuleucel-t (Provenge; Dendreon) and ipilimumab (Yervoy; Bristol-Myers Squibb). Although these agents comprise the first approvals for a new therapeutic class, their limited long-term benefit warrants development of more potent active immunotherapies. The oncolytic and immunotherapeutic herpes simplex virus T-Vec (Amgen), which expresses GM-CSF after local intratumoral injection, recently demonstrated anticancer immune induction and durable objective responses in an intratumoral phase 2 melanoma study1.
Oncolytic immunotherapies are designed to selectively replicate within, and subsequently lyse, cancer cells2,3,4,5 while inducing tumor-specific immunity. JX-594 (also called Pexa-Vec; Jennerex Inc.) is a vaccinia virus (Wyeth vaccine strain) with disruption of the viral thymidine kinase gene (TK) for cancer selectivity and insertion of human granulocyte-macrophage colony-stimulating factor (hGM-CSF) and β-galactosidase transgenes for immune stimulation and replication assessment, respectively6,7,8. JX-594 is designed to induce both virus replication–dependent oncolysis and tumor-specific immunity6,9,10. The advantages of a vaccinia virus include intravenous (i.v.) stability and delivery11, enhanced potency12, extensive safety experience as a live vaccine, demonstrated ability to induce efficient immune responses and a large transgene-encoding capacity6. The oncolytic immunotherapies JX-594 (Pexa-Vec), T-VEC (Amgen)13 and an adenoviral construct expressing GM-CSF (Ad-GM-CSF)3 comprise a new platform that has advantages over the currently approved immunotherapeutics given their direct tumor lysis, induction of subject tumor-specific immunity and use as off-the-shelf products.
In contrast to other agents in this class, JX-594 showed both complete responses of bulky tumors and systemic efficacy in phase 1 studies14,15. In a phase 1 clinical trial of intratumoral JX-594 (ref. 14), as can be expected with a self-amplifying agent, injection-site responses were seen at all doses; however, systemic tumor responses and delivery through the blood to distant tumors required a high dose. High-dose JX-594 was also required for i.v. delivery and efficacy in a dose-escalation i.v. phase 1 trial15. Thus far, however, no randomized dose-finding trial has been reported with these self-amplifying active immunotherapies. In addition, proof of active immunotherapy induction in subjects with these agents was lacking. The objectives of this randomized trial were to compare outcomes with low-dose (108 PFU) and high-dose (109 PFU) JX-594 in a uniform advanced solid tumor population (HCC), including safety, intrahepatic tumor response, induction of immunity to both cancer cells and vaccinia, and overall survival.
Results
Subject enrollment and baseline and treatment characteristics
Between December 2008 and May 2011, we screened 49 subjects for enrollment. Study enrollment was halted early by an independent data safety monitoring board because of a significant survival benefit favoring the high-dose group. We enrolled 30 subjects and stratified (viral or nonviral tumor etiology) and randomized them (16 high-dose arm and 14 low-dose arm; the trial profile is shown in Supplementary Fig. 1). Baseline subject characteristics and prognostic factors were well balanced between the two groups (Table 1). We noted no statistically significant differences for prognostic factors between groups. However, the high-dose subjects were more likely to have failed previous systemic therapy, which is a negative prognostic factor (with six high-dose subjects compared to one low-dose subject failing previous systemic therapy; P = 0.09, Fisher's exact test), including previous sorafenib treatment (all four subjects with previous sorafenib treatment in the high-dose group had tumor progression while on this therapy).
Twenty-nine subjects received all three doses; one subject received only two doses because of an unrelated adverse event. All subjects were evaluable for safety (as they all received at least one dose) and all but one (because of a major undocumented protocol deviation, biopsy-confirmed cholangiocarcinoma) were evaluable for survival. Twenty-eight subjects were considered evaluable for radiographic endpoints; this included two subjects who had clinical disease progression and died without a scan at week 8 (and were considered to have had progression). Therapies given after JX-594 treatment and off protocol, as reported by the principal investigators, were similar in the two groups; these included sorafenib treatment (full-dose sorafenib for ≥8 weeks in two high-dose subjects and one low-dose subject) and local-regional palliative therapies (two high-dose subjects and one low-dose subject).
Safety and toxicity
JX-594 was generally well tolerated at both doses. No treatment-related deaths were reported. One treatment-related serious adverse event was reported in the high-dose group (nausea and vomiting requiring prolonged hospitalization). Ten non–treatment-related serious adverse events were reported (in eight subjects, four high-dose and four low-dose). Treatment-related adverse events are summarized by grade and treatment arm in Supplementary Table 1. The frequency and grade (according to the National Cancer Institute Common Terminology Criteria for Adverse Events) of the adverse events were similar between the two dose groups. Flu-like symptoms (grade 1–2) occurred in all subjects over the first 12–24 h after treatment, including fever, rigors, nausea or vomiting. Subjects treated at the high dose showed a larger temperature increase after the first JX-594 treatment compared with low-dose subjects (P = 0.0023, t test) and had a higher incidence of anorexia (31% compared to 0%; P = 0.04). One possibly related grade 4 event of lymphopenia (2 week duration) was reported in a high-dose subject. Increases in serum transaminase concentrations were reported in six subjects (four low-dose and two high-dose).
A single high-dose subject developed eight to ten grade 1 skin pustules measuring <1 cm diameter each on the extremities, forehead and trunk. The lesions developed approximately 4 d after treatment and resolved completely without scar formation within approximately 6 weeks. The subject received two subsequent doses of JX-594 without delays.
Intrahepatic disease control and mRECIST and Choi responses
We performed serial dynamic magnetic resonance imaging (MRI) scans of the liver and abdomen, and these were subsequently read by expert independent central readers who were blinded to treatment arm. We applied the mRECIST response criteria developed for individuals with HCC16 to assess the effects of JX-594 treatment in the liver. In addition, as JX-594 has been shown to disrupt tumor blood flow and induce tumor necrosis17, we performed tumor contrast enhancement measurements according to the modified Choi criteria18 to assess effects on perfusion and the development of tumor necrosis.
As demonstrated in a previous study14, both doses were associated with intrahepatic antitumor activity. The intrahepatic mRECIST disease control rate at week 8 was 46% overall (28 evaluable subjects, with control rates of 47% and 46% for the high-dose and low-dose groups, respectively) and was 50% at any time point (meaning at week 8 or any other time, whichever was better). As the primary radiographic endpoint was at week 8, we did not consistently perform subsequent scans and as a result did not assess time to tumor progression.
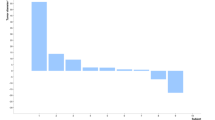
We found objective mRECIST responses and decreased tumor perfusion and contrast enhancement in both injected and noninjected tumors within both dose groups (Fig. 1a–d and Supplementary Fig. 2). In some cases, tumors with decreased contrast enhancement showed swelling and edema (Fig. 1e). The modified Choi response rate was 62% overall (26 evaluable subjects, with response rates of 57% and 67% for the high-dose and low-dose groups, respectively). The mean changes in the Choi parameter were −35.6% and −28.8% in the high-dose and low-dose groups, respectively (P = 0.73). Four objective mRECIST responses (one complete and three partial; Fig. 1f) and ten cases of stable disease were reported; dose did not correlate with intrahepatic response (Fig. 1a–d). The mean change in longest tumor diameter (mRECIST) may have reflected dose-related edema formation (changes of 23.8% and −8.7% in the high-dose and low-dose groups, respectively; P = 0.11). We found similar effects on tumor vascularity in non-injected tumors after high-dose treatment (Fig. 1g), including small masses (<1 cm) that were poorly visualized at baseline.
(a) Greatest decrease in the sum of the longest diameter (LD) of target tumors from baseline (mRECIST criteria) in livers of individual patients after JX-594 treatment. n = 22 patients who had measurable and evaluable tumors at baseline and at least one follow-up time point. A tumor with an increase >100% is indicated with a + above the bar. (b) Greatest decrease in tumor contrast enhancement or longest diameter from baseline (Choi criteria) in target tumors in livers of individual patients after JX-594 treatment. n = 24 patients who had measurable and evaluable tumors at baseline and at least one follow-up time point. A tumor with an increase >100% is indicated with a + above the bar. (c) Greatest decrease in the sum of the longest tumor diameter from baseline (RECIST criteria) in noninjected liver tumors of individual patients after JX-594 treatment. n = 8 evaluable. (d) Greatest decrease in tumor contrast enhancement or longest diameter from baseline (Choi criteria) in noninjected liver tumors of individual patients after JX-594 treatment. n = 8 evaluable. (e) Example of the effects of JX-594 on contrast enhancement and perfusion in an injected tumor (JX7-1401; high dose). (f) Example of the effects of JX-594 on the longest diameter of an injected tumor (complete mRECIST response) (JX7-1715; low dose). (g) Example of the effects of JX-594 on contrast enhancement and perfusion in a noninjected (distant) tumor (JX7-1403; high dose). The red circles (e–g) indicate the same (responding) tumors over time.
Pharmacokinetics, replication and transgene expression
JX-594 injections into tumors resulted in acute diffusion of JX-594 genomes into the blood, thus resulting in systemic distribution. We monitored the concentrations of JX-594 in the blood over time at 15 min, 3 h, 5–7 d and 14 d after completion of each injection procedure. The pharmacokinetic profiles were similar for all three injections per subject (Fig. 2a). Concentrations were highest at 15 min after injection; genomes were detectable in blood at this time point in all subjects on both arms. The peak concentrations of JX-594 were significantly greater for subjects in the high-dose arm (Fig. 2a); the mean concentration (± s.e.m.) for all cycles was 273,700 ± 55,650 genomes ml−1 for the high-dose group and 31,650 ± 5,317 genomes ml−1 for the low-dose group (P = 0.0002, t test). Acute blood concentrations at 15 min after injection were consistent with a substantial fraction of the input dose acutely entering the blood during or immediately after the injection procedure (half-life of ∼60 min). The peak concentrations in these subjects were similar to peak concentrations in subjects after i.v. infusion at similar doses in a phase 1 trial15; the peak concentrations in the high-dose group in this trial were above the threshold for i.v. delivery to tumors (as defined in the i.v. phase 1 trial), whereas the concentrations in the low-dose group were not. The frequency and concentrations of detectable JX-594 genomes in the blood were also significantly greater in the high-dose subjects at 3 h after injection (78% of subjects had detectable JX-594 compared to 45% for the high-dose and low-dose groups, respectively, for all treatment cycles; mean concentrations of 10,450 ± 2,090 genomes ml−1 and 2,098 ± 488 genomes ml−1 for the high-dose and low-dose groups, respectively, for all treatment cycles; P = 0.0006, t test).
(a) The mean (± s.e.m.) peak concentration of JX-594 (genomes measured by quantitative PCR (qPCR)) in blood after each treatment cycle (using blood obtained 15 min after the completion of treatment) by dose group (t test). IT, intratumoral. (b) The percentage of patients with evidence of β-gal transgene expression (+95% confidence interval (CI)) after JX-594 treatment (generation of antibodies (Ab) to the β-gal transgene product within 29 d of treatment is indicative of JX-594 replication, as β-gal protein expression is associated with virus replication) (Fisher's exact test). (c) The percentage of patients with evidence of hGM-CSF transgene expression (+95% CI) on day 5 after JX-594 treatment (Fisher's exact test). (d) Maximum induction of neutrophil concentration in blood after treatment cycle 1 by dose group (using blood obtained on days 5 and 15 after treatment). Black bars, low-dose JX-594; white bars, high-dose JX-594.
We assessed JX-594 replication and dual transgene expression using three methods. We first assessed antibody development to the β-galactosidase (β-gal) protein (the lacZ transgene product), which is dependent on viral replication and associated protein expression. Second, JX-594 expresses the hGM-CSF transgene under control of the synthetic early-late promoter such that high-level expression would occur during replication of the product in cancer cells6,7,8. Whereas i.v. JX-594 can acutely stimulate production of multiple cytokines, delayed hGM-CSF expression 5 or more days after infusion (when other cytokine concentrations have returned to baseline levels) was indicative of hGM-CSF expression from JX-594 in the context of replication14,15. Third, we evaluated the delayed re-emergence of JX-594 genomes in the blood on days 5, 15, 22, 29, 36, 43 and 57. The detection of JX-594 genomes in blood at any of these time points after JX-594 injection would suggest intratumoral replication and subsequent leakage into the systemic circulation. We were able to detect JX-594 genomes in the blood of three subjects (two high dose and one low dose) at the late time points (days 15–36).
As can be expected because of replication of JX-594 at both doses, β-gal–specific antibodies developed in most of the subjects during the treatment phase: 75% of high-dose and 62% of low-dose subjects developed these antibodies (Fig. 2b). Neutralizing antibody titers to vaccinia (and, hence, JX-594) were detectable at baseline (from childhood vaccination) in 50% of subjects; all subjects developed detectable titers by day 29.
hGM-CSF protein was quantifiable in the plasma on day 5 in 69% of the high-dose and 46% of the low-dose subjects (P = 0.27, Fisher's exact test; all baseline samples were negative for hGM-CSF) (Fig. 2c). Exploratory data from a subset of subjects treated with the same manufacturing lot of clinical trial material showed that hGM-CSF concentration (day 5) was higher in the high-dose subjects than in the low-dose subjects (median for the high-dose subjects was 47.6 pg ml−1 compared to 1.5 pg ml−1 for the low-dose subjects; P < 0.001, t test). Neutrophils and eosinophils are GM-CSF–responsive subsets of white blood cells. The absolute neutrophil and eosinophil concentrations increased substantially between 5–14 d after treatment in 65% of subjects with quantifiable hGM-CSF in the blood (Fig. 2d).
Induction of humoral and cellular anticancer immunity
We analyzed subject blood, tumor tissue and radiographic imaging to assess the induction of anticancer immunity (Fig. 3). As a measure of induction of antitumoral immunity, we assessed antibody-mediated complement-dependent cytotoxicity (CDC) in subjects' serum over time after treatment. Eleven of 16 (69%) subjects evaluated developed CDC against at least one of four HCC cell lines (six high-dose and five low-dose subjects) (Fig. 3a,b); CDC was evident even after serum dilution down to 5%. All HCC cell lines tested contained hepatitis B virus DNA except HepG2 (which is not virally associated); six subjects with CDC induction had hepatitis B–associated HCC, two had hepatitis C–associated HCC and three had non-viral HCC.
(a) Antibody-mediated complement-dependent cytotoxicity induction after JX-594 therapy in HCC (n = 4), normal (n = 2; HUVEC and MRC-5) and non-HCC (RCC, n = 3; melanoma, n = 4) cell lines. Each graph shows the mean percentage cell viability (+s.d.) after incubation with each individual patient's serum (diluted to 5%) collected on day 43 after the initiation of treatment compared to baseline. JX7-1704 and JX7-1702, two high-dose patients. (b) Antibody-mediated CDC induction against HCC cell lines in individual patients over time after JX-594 therapy (serum diluted to 5%; mean ± s.d.). The data shown are from serum of patients that induced ≤50% cell viability (>50% cell killing) on at least one follow-up time point. (c) Radiographic evidence of progressive necrosis and peripheral enhancement over time in noninjected tumors (JX7-0307; low dose). (d) H&E staining of a biopsy sample from a tumor collected from patient JX7-0301 (low dose) 1.5 years after the initiation of JX-594 treatment. Scale bars, 100 μm (low magnification); 50 μm (high-magnification inset). (e) Radiographic evidence of progressive necrosis and peripheral enhancement over time in a noninjected tumor (JX7-0301; low dose). Red circles (c,e) indicate the same (responding) tumors over time. (f) ELISPOT analysis detecting T cells producing interferon-γ in response to stimulation with β-gal peptides at baseline and after JX-594 treatment; data are expressed as the mean number of spot-forming cells (SFC) per 105 cells (+s.d.) (JX7-0310, low dose; JX7-0311, high dose). (−), negative control peptide. The P values in a and f were calculated by t test.
Radiographic evidence included progressive near-complete tumor hypovascularity and necrosis associated with a markedly enhancing rim in noninjected tumors developing over 3–4 months in four subjects (Fig. 3); these findings are considered highly unusual for tumor progression in HCC, but we could not completely rule out progression. We biopsied one of these masses 1.5 years after the last JX-594 treatment (Fig. 3e); the biopsy showed diffuse lymphocytic infiltration (Fig. 3d).
We also assessed cellular immunity. Cytotoxic T cells were induced to vaccinia peptides (data not shown) and the JX-594 transgene product β-gal (enzyme-linked immunosorbent spot (ELISPOT) analysis) (Fig. 3f); β-gal cytotoxic T cell activity was present in a high-dose subject 1.5 years after treatment initiation (Supplementary Fig. 3). In contrast, T cells collected from healthy donors not exposed to JX-594 did not have reactivity to β-gal (Supplementary Fig. 3).
Overall survival
The median overall survival was 9.0 months for the entire study population. We assessed the correlation between various baseline variables and overall survival (Table 2). Only dose cohort and peak JX-594 blood concentration correlated significantly with overall survival. Overall survival was significantly longer in the high-dose arm compared to in the low-dose arm (hazard ratio 0.39, P = 0.020, Gehan-Breslow-Wilcoxon test, one-sided test for superiority of the high dose). The median overall survival was 14.1 months for the high-dose group compared to 6.7 months for the low-dose group (Fig. 4a). Kaplan-Meier survival estimates for the high-dose and low-dose groups at 1 year were 66% and 23%, respectively, and at 18 months were 35% and 11%, respectively. Survival did not correlate with tumor etiology (viral or nonviral). In subjects with multiple tumors at baseline (ten high-dose and nine low-dose subjects), high-dose JX-594 was associated with a significant survival benefit; the median overall survival was 13.6 months in the high-dose group compared to 4.3 months in the low-dose group (hazard ratio 0.19, P = 0.018, Gehan-Breslow-Wilcoxon test, one-sided test for superiority of the high dose) (Fig. 4b). Subjects with multiple tumors (n = 19) had a median survival that was half that of subjects with single tumors (n = 10) in this trial (8.8 months compared to 16.6 months, respectively). The presence or absence of detectable neutralizing antibodies to vaccinia at baseline did not correlate with survival duration (hazard ratio of 0.68 in favor of baseline antibody-positive compared to antibody-negative subjects; P = 0.24, Gehan-Breslow-Wilcoxon test) (Fig. 4c). We performed an exploratory multivariate stepwise regression analysis to evaluate dose and peak genome concentration, neutrophil induction and antibody induction to the JX-594 β-gal transgene; as only 24 subjects were evaluable for all variables, this was only a hypothesis-generating analysis. This multivariate analysis confirmed that dose group was the best predictor of overall survival (χ2 = 2.0085, hazard ratio of 0.358); other variables did not add additional overall survival predictive value after dose.
(a) Overall survival in the entire evaluable study population (dashed line) and by dose group (n = 29 total). (b) Overall survival in subjects with multiple tumors at baseline (ten high-dose and nine low-dose subjects). (c) Overall survival in the entire evaluable study population by the presence or absence of baseline neutralizing antibody status. (d) Overall survival in high-dose subjects having previously failed systemic therapy (n = 6).
Six high-dose subjects had previously failed systemic therapy at the time of study enrollment, as described above; four in this group had failed previous treatment with sorafenib. The median survival for this subject population was estimated to be ∼2–4 months on the basis of data from randomized phase 3 trials with sorafenib19,20. High-dose subjects in this systemic therapy failure subgroup had a median survival of 13.6 months, and two such subjects were still alive after more than 2 years (Fig. 4d).
Discussion
We report here for the first time, to our knowledge, several key findings for JX-594 and the field of oncolytic immunotherapies. First, we demonstrated that JX-594 dose was an important determinant of overall survival duration in subjects with advanced carcinoma; given that oncolytic viruses replicate in tumor cells, it has been predicted that maximizing the dose to the subject would be less important than it is with other therapies in cancer. Second, to our knowledge, this is the first randomized clinical trial showing that an oncolytic virus or gene therapy agent was associated with significantly improved overall survival duration. Of note, the low dose in this trial had clear anticancer efficacy, and therefore, showing a significant survival impact for the high-dose group compared to low-dose active controls was presumably a higher hurdle than a comparison to placebo might have been. Third, we demonstrated the active induction of functional antitumor immunity with an oncolytic virus in multiple subjects within a homogenous population. JX-594 treatment induced a polyclonal humoral immune response resulting in antibody-dependent CDC. In a melanoma clinical trial with herpes simplex virus (HSV) hGM-CSF (T-Vec; Amgen), phenotypic analysis of T cells derived from tumor samples suggested distinct differences from peripheral blood T cells. Compared to T cells derived from nontreated control patients, there was an increase in melanoma-associated antigen recognized by T cells (MART-1)-specific T cells in tumors undergoing regression after vaccination; functional antitumoral immunity, however, was not assessed1.
These trial results address a number of key questions, but others remain unanswered. Although data on diverse MOA were obtained, including JX-594 replication and immune stimulation, the relative importance of each MOA remains to be determined. The only variable that correlated with overall survival duration, besides dose group, was acute peak JX-594 concentration in the blood; of note, blood concentrations in the high-dose group were above the threshold concentration required for i.v. delivery in phase 1 (ref. 15), whereas low-dose concentrations were not. Hence, these data suggest that systemic tumor control and improved survival may be achieved with JX-594 through high-dose i.v. administration. As predicted on the basis of this hypothesis, the survival benefit of high-dose JX-594 was most pronounced in patients with the most extensive tumor burdens. Replication and hGM-CSF transgene expression were associated with three MOA: acute vascular disruption in tumors, tumor oncolysis and necrosis and antitumor immunity. The long-term survival of high-dose patients (∼35% at 2 years) after dosing over only 4 weeks suggests a potential durable systemic benefit. Although not proven, this durable efficacy could reflect chronic viral oncolysis or immune-mediated effects, as demonstrated by tumor-lysing antibody generation and lymphocyte infiltration into tumors. Further study will be needed to assess the relative importance of each MOA in specific patient populations. Likewise, although helper T cell engagement was demonstrated by IgG class switching and the induction of T cells specific for the β-gal transgene, further data on tumor-specific T lymphocyte induction will need to be obtained in future trials. In addition, larger trials will allow the exploration of correlations between immune and virus-replication endpoints and patient survival. Choi response criteria include changes in tumor density and maximum diameter. The application of mRECIST and Choi criteria in HCC trials is increasing, and preliminary data suggest that these criteria may be better predictors of overall survival in HCC than the standard RECIST criteria21,22. Nevertheless, more data are needed to know how to interpret these mRECIST and Choi responses.
The survival analysis from this stratified and randomized clinical trial demonstrated a significant dose-dependent survival prolongation. However, these findings should be extended in larger randomized trials with a placebo control arm included. We selected the sample size of 30 patients to give sufficient power for toxicity analyses in both arms. Despite the relatively small sample size and an active control-group treatment, a statistically significant survival benefit was demonstrated because of the large effect size. Prognostic factors were well-balanced between the two arms, with the exception that more high-dose patients had the poor prognostic factor of having failed previous systemic therapy, including sorafenib treatment; the survival duration in this subgroup on high-dose therapy was similar to that of systemic treatment–naive patients. Therefore, the survival benefit of high-dose JX-594 cannot be explained by imbalances in known prognostic factors.
Large randomized trials adequately powered for overall survival effects are underway or have been planned with JX-594 in patients with advanced HCC. In a randomized trial involving patients having failed treatment with sorafenib, patients are being randomized to either JX-594 plus the best supportive (palliative) care or to the best supportive care alone (ClinicalTrials.gov, NCT01387555). Trials in other solid tumor populations are underway, including colorectal carcinomas with a mutant K-RAS genotype. Furthermore, targeted oncolytic vaccinia viruses such as JX-594 can be engineered to express diverse biologics with varied and potentially synergistic MOA; these include cytokines, tumor antigens, checkpoint inhibitors, prodrug-activating enzymes, single-chain antibodies and tumor antigens (reviewed in ref. 6). In particular, exploration of tumor antigen expression is warranted given our demonstration in this trial that a T cell response was induced to the JX-594 transgene β-gal. Given the large transgene-encoding capacity of vaccinia viruses, multiple tumor antigens, as well as complementary cytokines (for example, GM-CSF or interleukin-2 (IL-2)), could be expressed from the same virus backbone. In summary, the oncolytic and immunotherapeutic vaccinia virus JX-594 (Pexa-Vec) holds promise for the treatment of advanced solid tumors, and further clinical trials are warranted.
Methods
Study design.
This trial was a multicenter, multinational randomized and stratified, parallel-group dose-finding study in patients with advanced HCC. Patients were stratified (viral or nonviral) and randomized one-to-one to either the high-dose or low-dose cohort. The primary endpoint was assessed by independent expert radiologists who were blinded to treatment group; principal investigators were not blinded to dose group. All patients gave written informed consent according to the principles of Good Clinical Practice. The study protocol and consent forms were approved by the United States Food and Drug Administration, the Korea Food and Drug Administration and Health Canada, as well as the Institutional Review and Infection Control Committees at the following centers: Moores University of California San Diego (UCSD) Cancer Center, McMaster University Medical Center, Samsung Medical Center, Ohio State University, Severance Hospital Yonsei University Health System and Pusan National University Hospital. An independent data-safety monitoring board reviewed major safety assessments and periodically assessed safety and efficacy. The date of data cutoff for the survival analysis was the survival follow-up data collected when the last patient reached the week 8 primary endpoint assessment visit.
This study was designed by Jennerex Inc. in collaboration with the principal investigators. Data collection and monitoring were performed by Jennerex Inc. and Green Cross Corporation. Data management, safety reporting management, statistics, and radiographic image management, evaluation and analysis were performed by independent expert contractors. The manuscript was written and edited by Jennerex Inc. and the principal investigators together in collaboration with Green Cross Corporation after a joint decision to publish. The academic investigators vouch for the validity of the results.
Patients.
Inclusion criteria included unresectable and injectable (defined tumor margins) HCC (histologically confirmed or clinical and laboratory diagnosis defined according to the American Association for the Study of Liver Diseases guidelines), adequate hematological function (absolute neutrophil count >1,250 cells mm−3, white blood cell count >2,500 cells mm−3 and <50,000 cells mm−3, hemoglobin ≥9 g dl−1, platelet count ≥50,000 cells mm−3 and International Normalized Ratio (INR) ≤1.5 times the upper normal limit) and organ function (including serum creatinine <2 mg dl−1, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <5.0 times the upper normal limit, total bilirubin ≤2.5 times the upper normal limit and Child-Pugh score of A or B) and a KPS of 70 or greater. Exclusion criteria included liver tumors in a location that could result in clinical adverse effects as a result of tumor swelling after treatment (for example, tumors impinging on the biliary tract), known central nervous system malignancy, severe or unstable cardiac disease, symptomatic ascites, increased risk of vaccination complications (exfoliative skin conditions such as eczema or ectopic dermatitis), immunodeficiency caused by underlying illness, anticancer therapy within 4 weeks before the first treatment, use of certain antiviral agents with activity against vaccinia (for example, ribavirin, adefovir, cidofovir and PEG-IFN) and pregnancy or nursing. Patients with household contacts at known risk for vaccinia vaccine complications (for example, severe immune deficiency or eczema) who could not relocate were excluded. Portal vein thrombosis or invasion and extrahepatic disease were allowed. Three patients in this trial were included in a publication on the use of sorafenib after JX-594 treatment10.
Clinical trial material (CTM).
CTM was manufactured according to Good Manufacturing Practice guidelines. CTM was generated after infection of human carcinoma cells. Cells were harvested after approximately 48 h and lysed, and the cellular debris was removed by a standard filtration step. The remaining supernatant was purified by tangential flow filtration. Final-product quality-control tests included assays for sterility and endotoxin (absent), adventitious viruses (absent), DNA and protein content, potency measured in PFU ml−1 and hGM-CSF production. Immediately before the intratumoral (IT) injection, JX-594 was diluted in bicarbonate-buffered saline.
Randomization, masking and treatment.
After enrollment and stratification for HCC etiology (hepatitis B or C virus infection–associated or not virally associated), we randomized patients centrally using permuted blocks to receive a dose of 109 or 108 PFU distributed among up to five intrahepatic tumors on days 1, 15 and 29. We administered JX-594 by imaging-guided IT injection using a multi-pronged Quadrafuse needle (Rex Medical Inc) to ensure even distribution of the virus throughout the tumor when possible (if this was not technically feasible, a straight needle was used). The total dose per patient per treatment session was fixed at 109 or 108 PFU. The volume of JX-594 solution to be injected was proportional to the volume of the tumor to be injected (25% of the tumor volume).
Physical and laboratory assessments.
We performed physical assessments and interval medical histories at each visit over the first 8 weeks of study. Safety monitoring included adverse event monitoring according to the National Cancer Institute Common Toxicity Criteria (version 3.0) and standard laboratory toxicity grading for hematology, liver and renal function, coagulation studies, serum chemistry and urinalysis. We performed white blood cell counts by routine laboratory testing as defined in the protocol. We assessed white blood cell counts at baseline and on days 5, 15, 22, 29, 36, 43 and 57.
qPCR.
We evaluated the concentration of JX-594 genomes in blood over time using qPCR as previously described14,23. We collected samples before each injection and 15 min, 3 h and 5 d or 7 d after each treatment and on days 43 and 57 (2 and 4 weeks after the final treatment).
hGM-CSF ELISA.
We evaluated hGM-CSF protein concentrations in plasma at baseline and 5 d after the first treatment by solid-phase sandwich ELISA using the Quantikine hGM-CSF Kit (R&D Systems) as directed by the manufacturer.
β-gal–specific antibody ELISA.
We assessed antibody induction to β-gal at baseline and day 29. Human IgG antibodies to β-gal were measured by ELISA. Briefly, we incubated plates (NUNC MaxiSorp, Thermo Fisher Scientific) with wells containing β-gal (Sigma) or bicarbonate or carbonate buffer overnight at 4 °C and washed the plates with PBS-Tween before incubation with blocking buffer (PBS with 1% BSA, ELISA grade (Sigma)). We added diluted serum (1:50, 1:100 or 1:200 in PBS with 0.05% Tween and 1% BSA) to β-gal–coated and control wells in duplicate and incubated them at 23 °C. We washed the plates and incubated them with alkaline-phosphatase–labeled goat human-specific IgG (Abcam) diluted 1:2,000. After washing, we added the colorimetric substrate alkaline phosphatase yellow (pNPP, Sigma) and added NaOH 10 min later to stop color development. We read the absorbance at 405 nm and subtracted the absorbance at 630 nm. We subtracted control-well values to account for nonspecific binding and calculated titer values by comparison to a standard curve of positive sera, arbitrarily assigned a titer of 8,000.
Detection of neutralizing antibodies.
We measured JX-594–specific neutralizing antibody titers at baseline and on days 5, 15, 29 and 57 by cytopathic effect inhibition assay as previously described14,15. Titers are expressed as the reciprocal of the highest dilution (least concentrated) of heat-inactivated serum that resulted in ≥50% cell viability; the lowest dilution tested was 10%, hence limit of detection of the assay was 10. We measured β-gal–specific antibody titers at baseline and on day 29 by ELISA as previously described15.
Histopathology analysis.
We obtained core-needle biopsies of liver tumors in a subset of patients after treatment. The tissue was formalin fixed and paraffin embedded. We stained sections with H&E to assess tissue histology, necrosis and inflammation.
Radiology acquisition and analysis.
We performed multiphase dynamic contrast-enhanced MRI of the abdomen on either a 1.5T or 3.0T MR system using extracellular gadolinium chelate contrast agent. We performed scans at baseline (days −14 to 0) and at week 8; scans were optional every 6 weeks thereafter. Scans were evaluated by two independent radiologists with expertise in liver cancer assessment (R.P. and R.L.); the readers were blinded to treatment group. Given the mechanism of tumor destruction on the basis of vascular disruption previously described for JX-594 (ref. 14), we elected to assess tumor vascularity (contrast enhancement) at baseline and at each time point using both the mRECIST for HCC criteria16 (performed by R.L.) and a modification of Choi response assessments24 (performed by R.P.). Choi response criteria include tumor decreases from baseline of >10% in longest diameter or >15% decrease in tumor density; these criteria were modified compared to the standard published Choi criteria because standard Choi uses computed tomography scans rather than MRI scans. Twenty-eight patients were evaluable for overall mRECIST response: 2 patients died before the week 8 efficacy assessment and were categorized as progressive disease, 4 patients were evaluable by mRECIST but did not have measurable tumors, and 22 patients had measurable and evaluable disease. Twenty-six patients were evaluable for overall Choi response: 2 patients died before the week 8 efficacy assessment and were categorized as nonresponders, and 24 patients had measurable tumors. Two patients who were evaluable for mRECIST longest diameter changes were not evaluable for contrast enhancement changes.
Complement-dependent cytotoxicity assay.
SNU349, SNU482 and SNU267 (human renal cell carcinoma; obtained from Korean Cell Line Bank (KCLB)) and SNU475, SNU398, SNU449 and SNU739 (human hepatocellular carcinoma, hepatitis-B associated; obtained from KCLB) cells were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS (Hyclone) with penicillin and streptomycin. HepG2 cells (human hepatocellular carcinoma, not virally associated; obtained from ATCC) were cultured in DMEM medium containing 10% FBS with penicillin and streptomycin. MRC-5 nontransformed cells (lung fibroblast; obtained from KCLB) were cultured in minimum essential medium (MEM) containing 10% FBS with penicillin and streptomycin. HUVEC (endothelial cells; Lonza) were cultured in the endothelial cell medium EBM-2 (Lonza, MD, USA) supplemented with 2% FBS with penicillin and streptomycin. SK-MEL-2, SK-MEL-5 and WM-266-4 cells (human melanoma cells; obtained from KCLB) were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS (Hyclone) with penicillin and streptomycin. Lox IMVI cells (human melanoma cells; ATCC) were cultured in MEM containing 10% FBS with penicillin and streptomycin.
We assessed CDC activity by measuring cell viability after incubation with 5% serum in 96-well plates. We normalized cell viability in serum after JX-594 administration to the cell viability of patient serum at baseline (before JX-594 treatment). We seeded each cell line onto 96-well plates and incubated the cells overnight. We subsequently incubated cells with DMEM (no FBS) and the serum sample at 37 °C for 4 h. We then exposed cells to PBS and 10 μl Cell counting kit-8 (CCK-8) solution (CCK-8 kit, Dojindo, Inc.) and incubated cells at 37 °C for 2 h. Cell viability was measured by optical density at 450 nm.
β-gal–specific T cell analysis.
We drew peripheral blood mononuclear cells (PBMCs) from two patients before and after treatment with JX-594 and stimulated them with autologous dendritic cells pulsed with an overlapping peptide library (20-mer amino acids overlapping by 15 amino acids) spanning the entire protein sequence of β-gal. We also drew PBMCs 1.5 years after the last JX-594 dose in an additional patient. We also isolated PBMCs from four healthy donor controls. We maintained T cells in T cell medium (TCM) (RPMI 1640 (Hyclone) supplemented with 45% Click's medium (Irvine Scientific), 2 mmol l−1 GlutaMAX TM-I (Invitrogen) and 5% Human AB Serum (Valley Biomedical, Winchester, VA)) supplemented with 1,000 U ml−1 IL-4 (R&D Systems, Minneapolis, MN) and 10 ng ml−1 IL-7 (PeproTech). We harvested cells on day 9 and tested for β-gal specificity using ELISPOT assay.
We used ELISPOT analysis as a semiquantitative measure of antigen-specific effector T cells as previously described25. Briefly, we seeded 2.5 × 104 to 105 T cells in triplicate with individual pepmixes spanning β-gal at 0.1 μg per peptide per well. We used a pepmix of a testis cancer antigen, NY-ESO1, at 0.1 μg per peptide per well and PHA (phytohemagluttinin) at 2 μl (1 mg ml−1) as negative and positive controls, respectively. After 18 h of incubation, we developed the plates and sent them to Zellnet Consulting, NJ for quantification. SFC counts and input cell numbers were plotted, and a linear regression was calculated after excluding plateau data points. We expressed the frequency of T cells specific to each antigen as specific SFC per input cell numbers.
Statistical analyses.
The trial was designed to enroll 30 patients evaluable for safety and radiographic endpoints. The primary objective was intrahepatic disease control rate (mRECIST complete or partial response or stable disease) at week 8. Secondary objectives included safety and antitumor activity (change in contrast enhancement, mRECIST for HCC); pharmacokinetics, immune parameters and overall survival were other endpoints. All patients who received at least one dose of JX-594 were evaluable for safety. All patients who received at least one dose and had no major documented protocol deviations were evaluable for overall survival; patients with evaluable baseline and week 8 scans were evaluable for radiographic efficacy endpoints. By protocol, 15 evaluable patients were to be enrolled at each dose to test the hypothesis that the true success rate was at most 33% (meaning little or no activity) versus the alternative hypothesis that the true success rate was 66% or greater independently in each treatment arm (78% power, one-sided type I error rate of 0.03). Overall survival was assessed by Kaplan-Meier analysis26, and comparisons were made using the Gehan-Breslow-Wilcoxon test and a one-sided α for the statistical significance of high-dose superiority. A one-sided P value was appropriate for this dose-finding study because the objective was to determine whether the high dose was more effective for tumor control and overall survival to justify its potentially higher risk of side effects. The Gehan-Breslow-Wilcoxon test was selected because dosing occurred only over the first 4 weeks, and therefore a test that weighted the early part of the overall survival curves more heavily was appropriate and justifiable. Dichotomous variables were evaluated by Fisher's exact test, whereas continuous variables were compared by t test using the GraphPad Prism 5.0 software (GraphPad Software).
Role of the funding source.
The sponsor of the study participated in study design, data collection, data analysis, data interpretation and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
Kaufman, H.L. et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 17, 718–730 (2010).
Kirn, D., Martuza, R.L. & Zwiebel, J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7, 781–787 (2001).
Liu, T.C., Galanis, E. & Kirn, D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 4, 101–117 (2007).
Chiocca, E.A. Oncolytic viruses. Nat. Rev. Cancer 2, 938–950 (2002).
Heise, C. & Kirn, D.H. Replication-selective adenoviruses as oncolytic agents. J. Clin. Invest. 105, 847–851 (2000).
Kirn, D.H. & Thorne, S.H. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer 9, 64–71 (2009).
Kim, J.H. et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 14, 361–370 (2006).
Parato, K.A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 20, 749–758 (2012).
Hwang, T.H. et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. 19, 1913–1922 (2011).
Heo, J. et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol. Ther. 19, 1170–1179 (2011).
Thorne, S.H. et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Invest. 117, 3350–3358 (2007).
Wein, L.M., Wu, J.T. & Kirn, D.H. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res. 63, 1317–1324 (2003).
Senzer, N.N. et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor–encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 27, 5763–5771 (2009).
Park, B.H. et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 9, 533–542 (2008).
Breitbach, C.J. et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477, 99–102 (2011).
Lencioni, R. & Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 30, 52–60 (2010).
Liu, T.C., Hwang, T., Park, B.H., Bell, J. & Kirn, D.H. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 16, 1637–1642 (2008).
Choi, H. et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J. Clin. Oncol. 25, 1753–1759 (2007).
Llovet, J.M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Cheng, A.L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Faivre, S. et al. Changes in tumor density in patients with advanced hepato-cellular carcinoma treated with sunitinib. Clin. Cancer Res. 17, 4504–4512 (2011).
Edeline, J. et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer 118, 147–156 (2012).
Kulesh, D.A. et al. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the roche LightCycler and the Cepheid smart Cycler platforms. J. Clin. Microbiol. 42, 601–609 (2004).
Choi, H. et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am. J. Roentgenol. 183, 1619–1628 (2004).
Gerdemann, U. et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol. Ther. 20, 1622–1632 (2012).
Kaplan, E.L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 471–481 (1958).
Acknowledgements
J.-M. Limacher, M. Homerin, B.M. Bastien and M. Lusky (all from Transgene SA) gave insightful comments on the manuscript. T.-H.H. and M.K.K. were supported by a grant of the Korea Healthcare technology Research and Development Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A091047). C.R. was supported by a pilot grant from the Dan Duncan Cancer Center. M.N. was supported by the Robert and Janice McNair Foundation and Baylor Research Advocates for Student Scientists Fund. J.C.B. is supported by the Ontario Institute for Cancer Research and the Terry Fox Foundation. Funding was provided by Jennerex, Transgene SA (Illkirch, France) and the Green Cross Corporation; grants to T.-H.H. from Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea; to J.C.B. from the Terry Fox Foundation and the Canadian Institute for Health Research (CIHR); and a pilot grant to C.R. from the Dan Duncan Cancer Center. This trial was registered with ClinicalTrials.gov, number NCT00554372.
Author information
Authors and Affiliations
Contributions
D.H.K. and R.P. designed the study. C.J.B., D.H.K., T.-H.H., A.M., R.P., T.H., K.D., J.C.B., R.L., L.L., B.-G.R., M.C., C.R. and J.B. analyzed the data and wrote the manuscript. Y.S.L., M.K.K., M.D. and M.N. performed bioanalytical analyses. J.H., T.R., L.R., S.R., M.B., H.Y.L., H.C.C. and C.W.K. enrolled and managed the patients. C.J.B. and D.H.K. had access to all the data in the trial. D.H.K. made the final decision to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
C.J.B., J.C.B., A.M., K.D., L.L. and D.H.K. are employees of Jennerex, Inc. R.L., M.D., R.P., J.C.B. and T.-H.H. consult for Jennerex, Inc. J.H. and M.C. have received travel grants from Jennerex, Inc. B.-G.R. is an employee of Green Cross, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Table 1 (PDF 350 kb)
Rights and permissions
About this article
Cite this article
Heo, J., Reid, T., Ruo, L. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19, 329–336 (2013). https://doi.org/10.1038/nm.3089
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3089
This article is cited by
-
Synergistic combination therapy using cowpea mosaic virus intratumoral immunotherapy and Lag-3 checkpoint blockade
Cancer Immunology, Immunotherapy (2024)
-
Oncolytic virotherapy evolved into the fourth generation as tumor immunotherapy
Journal of Translational Medicine (2023)
-
Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment
Cell Communication and Signaling (2023)
-
Evolving therapeutic landscape of advanced hepatocellular carcinoma
Nature Reviews Gastroenterology & Hepatology (2023)
-
Oncolytic virotherapy: basic principles, recent advances and future directions
Signal Transduction and Targeted Therapy (2023)