Abstract
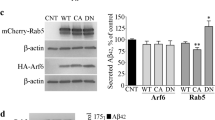
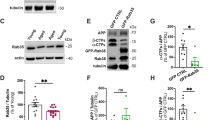
β-arrestins are associated with numerous aspects of G protein–coupled receptor (GPCR) signaling and regulation and accordingly influence diverse physiological and pathophysiological processes. Here we report that β-arrestin 2 expression is elevated in two independent cohorts of individuals with Alzheimer's disease. Overexpression of β-arrestin 2 leads to an increase in amyloid-β (Aβ) peptide generation, whereas genetic silencing of Arrb2 (encoding β-arrestin 2) reduces generation of Aβ in cell cultures and in Arrb2−/− mice. Moreover, in a transgenic mouse model of Alzheimer's disease, genetic deletion of Arrb2 leads to a reduction in the production of Aβ40 and Aβ42. Two GPCRs implicated previously in Alzheimer's disease (GPR3 and the β2-adrenergic receptor) mediate their effects on Aβ generation through interaction with β-arrestin 2. β-arrestin 2 physically associates with the Aph-1a subunit of the γ-secretase complex and redistributes the complex toward detergent-resistant membranes, increasing the catalytic activity of the complex. Collectively, these studies identify β-arrestin 2 as a new therapeutic target for reducing amyloid pathology and GPCR dysfunction in Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ballatore, C., Lee, V.M. & Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 (2007).
Götz, J., Ittner, A. & Ittner, L.M. Tau-targeted treatment strategies in Alzheimer's disease. Br. J. Pharmacol. 165, 1246–1259 (2012).
Holtzman, D.M., Goate, A., Kelly, J. & Sperling, R. Mapping the road forward in Alzheimer's disease. Sci. Transl. Med. 3, 114ps148 (2011).
Golde, T.E., Schneider, L.S. & Koo, E.H. Anti-aβ therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron 69, 203–213 (2011).
Karran, E., Mercken, M. & De Strooper, B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712 (2011).
Cramer, P.E. et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506 (2012).
Selkoe, D.J. Resolving controversies on the path to Alzheimer's therapeutics. Nat. Med. 17, 1060–1065 (2011).
De Strooper, B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-Secretase complex. Neuron 38, 9–12 (2003).
Bertram, L., Lill, C.M. & Tanzi, R.E. The genetics of Alzheimer disease: back to the future. Neuron 68, 270–281 (2010).
Bekris, L.M., Yu, C.E., Bird, T.D. & Tsuang, D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 23, 213–227 (2010).
Morris, J.C. et al. APOE predicts amyloid-β but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67, 122–131 (2010).
Fredriksson, R. & Schioth, H.B. The repertoire of G-protein–coupled receptors in fully sequenced genomes. Mol. Pharmacol. 67, 1414–1425 (2005).
Gudermann, T., Nurnberg, B. & Schultz, G. Receptors and G proteins as primary components of transmembrane signal transduction. Part 1. G-protein–coupled receptors: structure and function. J. Mol. Med. 73, 51–63 (1995).
Watson, S.A.S. The G Protein-Coupled Receptor Factors Book (Academic, San Diego, 1994).
Vassilatis, D.K. et al. The G protein–coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. USA 100, 4903–4908 (2003).
Thathiah, A. & De Strooper, B. The role of G protein–coupled receptors in the pathology of Alzheimer's disease. Nat. Rev. Neurosci. 12, 73–87 (2011).
DeWire, S.M., Ahn, S., Lefkowitz, R.J. & Shenoy, S.K. β-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 (2007).
Whalen, E.J., Rajagopal, S. & Lefkowitz, R.J. Therapeutic potential of β-arrestin– and G protein–biased agonists. Trends Mol. Med. 17, 126–139 (2011).
Beaulieu, J.M. et al. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273 (2005).
Luan, B. et al. Deficiency of a β-arrestin–2 signal complex contributes to insulin resistance. Nature 457, 1146–1149 (2009).
Beaulieu, J.M. et al. A β-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132, 125–136 (2008).
Shearman, M.S. et al. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid β-protein precursor γ-secretase activity. Biochemistry 39, 8698–8704 (2000).
Conner, D.A. et al. β-arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to β-adrenergic stimulation. Circ. Res. 81, 1021–1026 (1997).
Bohn, L.M. et al. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science 286, 2495–2498 (1999).
Ferguson, S.S. et al. Role of β-arrestin in mediating agonist-promoted G protein–coupled receptor internalization. Science 271, 363–366 (1996).
Lohse, M.J., Lefkowitz, R.J., Caron, M.G. & Benovic, J.L. Inhibition of β-adrenergic receptor kinase prevents rapid homologous desensitization of β 2-adrenergic receptors. Proc. Natl. Acad. Sci. USA 86, 3011–3015 (1989).
Ahn, S., Shenoy, S.K., Wei, H. & Lefkowitz, R.J. Differential kinetic and spatial patterns of β-arrestin and G protein–mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 279, 35518–35525 (2004).
Luttrell, L.M. et al. β-arrestin–dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661 (1999).
Thathiah, A. et al. The orphan G protein–coupled receptor 3 modulates amyloid-β peptide generation in neurons. Science 323, 946–951 (2009).
Ni, Y. et al. Activation of β2-adrenergic receptor stimulates γ-secretase activity and accelerates amyloid plaque formation. Nat. Med. 12, 1390–1396 (2006).
Teng, L., Zhao, J., Wang, F., Ma, L. & Pei, G.A. GPCR/secretase complex regulates β- and γ-secretase specificity for Aβ production and contributes to AD pathogenesis. Cell Res. 20, 138–153 (2010).
Olson, K.R. & Eglen, R.M. β galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev. Technol. 5, 137–144 (2007).
Gáborik, Z. et al. The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology 144, 2220–2228 (2003).
Shenoy, S.K. et al. β-arrestin–dependent, G protein–independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 281, 1261–1273 (2006).
Wei, H. et al. Independent β-arrestin 2 and G protein–mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. USA 100, 10782–10787 (2003).
De Strooper, B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998).
Mitani, Y. et al. Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein–transgenic and nontransgenic mice. J. Neurosci. 32, 2037–2050 (2012).
Chini, B. & Parenti, M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J. Mol. Endocrinol. 32, 325–338 (2004).
Vetrivel, K.S. et al. Association of γ-secretase with lipid rafts in post-Golgi and endosome membranes. J. Biol. Chem. 279, 44945–44954 (2004).
Wada, S. et al. γ-secretase activity is present in rafts but is not cholesterol-dependent. Biochemistry 42, 13977–13986 (2003).
Wahrle, S. et al. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9, 11–23 (2002).
Yagishita, S., Morishima-Kawashima, M., Ishiura, S. & Ihara, Y. Aβ46 is processed to Aβ40 and Aβ43, but not to Aβ42, in the low density membrane domains. J. Biol. Chem. 283, 733–738 (2008).
Chun, J., Yin, Y.I., Yang, G., Tarassishin, L. & Li, Y.M. Stereoselective synthesis of photoreactive peptidomimetic γ-secretase inhibitors. J. Org. Chem. 69, 7344–7347 (2004).
Chau, D.M., Crump, C.J., Villa, J.C., Scheinberg, D.A. & Li, Y.M. Familial Alzheimer disease presenilin-1 mutations alter the active site conformation of γ-secretase. J. Biol. Chem. 287, 17288–17296 (2012).
Vetrivel, K.S. et al. Spatial segregation of γ-secretase and substrates in distinct membrane domains. J. Biol. Chem. 280, 25892–25900 (2005).
Li, Y.M. et al. Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc. Natl. Acad. Sci. USA 97, 6138–6143 (2000).
Esler, W.P. et al. Activity-dependent isolation of the presenilin–γ-secretase complex reveals nicastrin and a γ substrate. Proc. Natl. Acad. Sci. USA 99, 2720–2725 (2002).
Fraering, P.C. et al. Detergent-dependent dissociation of active γ-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry 43, 323–333 (2004).
Radde, R. et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7, 940–946 (2006).
Poulin, B. et al. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. USA 107, 9440–9445 (2010).
Bohn, L.M., Gainetdinov, R.R., Lin, F.T., Lefkowitz, R.J. & Caron, M.G. Mu-opioid receptor desensitization by β-arrestin–2 determines morphine tolerance but not dependence. Nature 408, 720–723 (2000).
De Strooper, B. et al. A presenilin-1–dependent γ-secretase–like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999).
Annaert, W.G. et al. Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron 32, 579–589 (2001).
Esselens, C. et al. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J. Cell Biol. 166, 1041–1054 (2004).
Cai, H. et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 4, 233–234 (2001).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Bossers, K. et al. Concerted changes in transcripts in the prefrontal cortex precede neuropathology in Alzheimer's disease. Brain 133, 3699–3723 (2010).
Hébert, S.S. et al. Coordinated and widespread expression of γ-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol. Dis. 17, 260–272 (2004).
Acknowledgements
We are grateful to R.J. Lefkowitz and S. Ahn (Duke University Medical Center, Durham, North Carolina, USA) for the generous gift of the β-arrestin 2 wild-type and knockout mouse embryonic fibroblasts, the Arrb1−/− and Arrb2−/− mice, the β-arrestin 2–GFP-Flag cDNA and helpful discussion. We thank M. Jucker (University of Tübingen, Germany) for the gift of APP/PS1 transgenic mice. We greatly appreciate the kind gift of human control and Alzheimer's disease brain samples from K. Bossers and D.F. Swaab (Netherlands Institute for Neuroscience, Amsterdam, The Netherlands) and C. Troakes (the London Neurodegenerative Diseases Brain Bank, London, UK). We thank M. Mercken (Johnson & Johnson Pharmaceuticals Research and Development, Beerse, Belgium) for the antibodies to Aβ. We are grateful to Y. Li (Memorial Sloan Kettering Cancer Center, New York, USA) for the kind initial gift of JC-8. This work was supported by a Mentored New Investigator Research grant from the Alzheimer's Association to A.T., the Fund for Scientific Research Flanders, KU Leuven, a Methusalem grant from the KU Leuven and the Flemish government, and the Foundation for Alzheimer Research (SAO/FRMA) to B.D.S. B.D.S. is the Arthur Bax and Anna Vanluffelen chair for Alzheimer's disease.
Author information
Authors and Affiliations
Contributions
A.T. and B.D.S. designed the experiments and wrote the manuscript. A.T., K.H., A.S., E.V. and Y.H. conducted the experiments. M.C. conducted the qPCR experiments. G.D.K. synthesized JC-8. S.M. conducted the immunofluorescence image analysis.
Corresponding authors
Ethics declarations
Competing interests
B.D.S. receives research funding from and is a consultant for Janssen Pharmaceutica, Beerse, Belgium, Envivo, and Remynd, Leuven, Belgium. A.T. and B.D.S. are inventors on a patent that links β-arrestin to GPR3 and the γ-secretase complex, which is owned by VIB.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1391 kb)
Rights and permissions
About this article
Cite this article
Thathiah, A., Horré, K., Snellinx, A. et al. β-arrestin 2 regulates Aβ generation and γ-secretase activity in Alzheimer's disease. Nat Med 19, 43–49 (2013). https://doi.org/10.1038/nm.3023
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3023
This article is cited by
-
The multifaceted functions of β-arrestins and their therapeutic potential in neurodegenerative diseases
Experimental & Molecular Medicine (2024)
-
β-arrestin1 regulates astrocytic reactivity via Drp1-dependent mitochondrial fission: implications in postoperative delirium
Journal of Neuroinflammation (2023)
-
Adrenergic receptors blockade alleviates dexamethasone-induced neurotoxicity in adult male Wistar rats: Distinct effects on β-arrestin2 expression and molecular markers of neural injury
DARU Journal of Pharmaceutical Sciences (2023)
-
Characterization of behavioral changes in T-maze alternation from dopamine D1 agonists with different receptor coupling mechanisms
Psychopharmacology (2023)
-
Plasma Exo-miRNAs Correlated with AD-Related Factors of Chinese Individuals Involved in Aβ Accumulation and Cognition Decline
Molecular Neurobiology (2022)