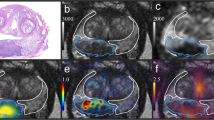
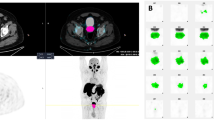
Abstract
A noninvasive technology that quantitatively measures the activity of oncogenic signaling pathways could have a broad impact on cancer diagnosis and treatment with targeted therapies. Here we describe the development of 89Zr-desferrioxamine–labeled transferrin (89Zr-transferrin), a new positron emission tomography (PET) radiotracer that binds the transferrin receptor 1 (TFRC, CD71) with high avidity. The use of 89Zr-transferrin produces high-contrast PET images that quantitatively reflect treatment-induced changes in MYC-regulated TFRC expression in a MYC-driven prostate cancer xenograft model. Moreover, 89Zr-transferrin imaging can detect the in situ development of prostate cancer in a transgenic MYC prostate cancer model, as well as in prostatic intraepithelial neoplasia (PIN) before histological or anatomic evidence of invasive cancer. These preclinical data establish 89Zr-transferrin as a sensitive tool for noninvasive measurement of oncogene-driven TFRC expression in prostate and potentially other cancers, with prospective near-term clinical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gatter, K.C., Brown, G., Trowbridge, I.S., Woolston, R.E. & Mason, D.Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J. Clin. Pathol. 36, 539–545 (1983).
Neckers, L.M. & Trepel, J.B. Transferrin receptor expression and the control of cell growth. Cancer Invest. 4, 461–470 (1986).
Richardson, D.R. Therapeutic potential of iron chelators in cancer therapy. Adv. Exp. Med. Biol. 509, 231–249 (2002).
Taetle, R., Honeysett, J.M. & Trowbridge, I. Effects of anti-transferrin receptor antibodies on growth of normal and malignant myeloid cells. Int. J. Cancer 32, 343–349 (1983).
Weiner, R.E. The mechanism of 67Ga localization in malignant disease. Nucl. Med. Biol. 23, 745–751 (1996).
Högemann-Savellano, D. et al. The transferrin receptor: a potential molecular imaging marker for human cancer. Neoplasia 5, 495–506 (2003).
O'Donnell, K.A. et al. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol. Cell. Biol. 26, 2373–2386 (2006).
Ellwood-Yen, K. et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4, 223–238 (2003).
Tacchini, L., Bianchi, L., Bernelli-Zazzera, A. & Cairo, G. Transferrin receptor induction by hypoxia. J. Biol. Chem. 274, 24142–24146 (1999).
Galvez, T. et al. siRNA screen of the human signaling proteome identifies the PtdIns(3,4,5)P3-mTOR signaling pathway as a primary regulator of transferrin uptake. Genome Biol. 8, R142 (2007).
Larson, S.M. Mechanisms of localization of gallium-67 in tumors. Semin. Nucl. Med. 8, 193–203 (1978).
Som, P. et al. 97Ru-transferrin uptake in tumor and abscess. Eur. J. Nucl. Med. 8, 491–494 (1983).
Vavere, A.L. & Welch, M.J. Preparation, biodistribution, and small animal PET of 45Ti-transferrin. J. Nucl. Med. 46, 683–690 (2005).
Lee, S.-I. et al. Molecular scintigraphic imaging using 99mTc–transferrin is useful for early detection of synovial inflammation of collagen-induced arthritis mouse. Rheumatol. Int. 29, 153–157 (2008).
Prost, A.C. et al. Tissue distribution of 131I radiolabeled transferrin in the athymic nude mouse: localization of a human colon adenocarcinoma HT-29 xenograft. Int. J. Rad. Appl. Instrum. B 17, 209–216 (1990).
Aloj, L. et al. Targeting of transferrin receptors in nude mice bearing A431 and LS174T xenografts with [18F]holo-transferrin: permeability and receptor dependence. J. Nucl. Med. 40, 1547–1555 (1999).
Holland, J.P., Sheh, Y. & Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 36, 729–739 (2009).
Holland, J.P., Williamson, M.J. & Lewis, J.S. Unconventional nuclides for radiopharmaceuticals. Mol. Imaging 9, 1–20 (2010).
Holland, J.P. et al. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 51, 1293–1300 (2010).
Holland, J.P. & Lewis Jason, S. Zirconium-89 chemistry in the design of novel radiotracers for immuno-PET. in Technetium and Other Radiometals in Chemistry and Medicine (eds. Mazzi, U., Eckelman, W.C. & Volkert, W.A.) 187–192 (Servizi Grafici Editoriali snc, Padova, Italy, 2010).
Meijs, W.E., Herscheid, J.D.M., Haisma, H.J. & Pinedo, H.M. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Rad. Appl. Instrum. A 43, 1443–1447 (1992).
Verel, I. et al. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 44, 1271–1281 (2003).
Daniels, T.R., Delgado, T., Rodriguez, J.A., Helguera, G. & Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 121, 144–158 (2006).
Haynes, B.F. et al. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J. Immunol. 126, 1409–1414 (1981).
Gotthardt, M., Bleeker-Rovers, C.P., Boerman, O.C. & Oyen, W.J.G. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J. Nucl. Med. 51, 1937–1949 (2010).
Ohkubo, Y., Kohno, H., Suzuki, T. & Kubodera, A. Relation between 67Ga uptake and the stage of inflammation induced by turpentine oil in rats. Radioisotopes 34, 7–10 (1985).
Heneweer, C., Holland, J.P., Divilov, V., Carlin, S. & Lewis, J.S. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J. Nucl. Med. 52, 623–633 (2011).
Watson, P.A. et al. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 65, 11565–11571 (2005).
Aloj, L., Carson, R.E., Lang, L., Herscovitch, P. & Eckelman, W.C. Measurement of transferrin receptor kinetics in the baboon liver using dynamic positron emission tomography imaging and [18F]holo-transferrin. Hepatology 25, 986–990 (1997).
Aloj, L., Lang, L., Jagoda, E., Neumann, R.D. & Eckelman, W.C. Evaluation of human transferrin radiolabeled with N-succinimidyl 4-[fluorine-18](fluoromethyl) benzoate. J. Nucl. Med. 37, 1408–1412 (1996).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Pellegrino, D. et al. Inflammation and infection: imaging properties of 18F-FDG–labeled white blood cells versus 18F-FDG. J. Nucl. Med. 46, 1522–1530 (2005).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Delmore, J.E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528 (2011).
Abe, N., Inoue, T., Galvez, T., Klein, L. & Meyer, T. Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor. J. Cell Sci. 121, 1488–1494 (2008).
Kim, S. et al. Regulation of transferrin recycling kinetics by PtdIns[4,5]P2 availability. FASEB J. 20, 2399–2401 (2006).
Gayed, I. et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J. Nucl. Med. 45, 17–21 (2004).
Holland, J.P. et al. Measuring the pharmacokinetic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using 89Zr-DFO-trastuzumab. PLoS ONE 5, e8859 (2010).
Beattie, B.J. et al. Multimodality registration without a dedicated multimodality scanner. Mol. Imaging 6, 108–120 (2007).
Acknowledgements
We thank N. Pillarsetty, B. Carver, D. Ulmert and P. Zanzonico for informative discussions, V. Longo for assistance with the in vivo studies, and M. Balbas for help with in vitro experiments. We thank C. Le and D. Winkleman for recording the MRI data and B. Beattie for assistance with co-registering PET/CT data. We thank M. McDevitt for assistance with HPLC stability studies. We also thank the staff of the Radiochemistry and Cyclotron Core at MSKCC. Funded in part by the Geoffrey Beene Cancer Research Center of MSKCC (J.S.L.), the Office of Science (BER)–US Department of Energy (Award DE-SC0002456, J.S.L.) and the R25T Molecular Imaging for Training in Oncology Program (2R25-CA096945; principal investigator H. Hricak; fellows: M.J.E. and S.L.R.) from the US National Cancer Institute. Technical services provided by the MSKCC Small-Animal Imaging Core Facility were supported in part by the US National Institutes of Health (NIH) grant R24-CA83084; NIH Center grant P30-CA08748; and NIH Prostate SPORE, P50-CA92629.
Author information
Authors and Affiliations
Contributions
J.P.H conducted all chemistry and radiochemistry. M.J.E. conducted all cellular assays. J.P.H., M.J.E., S.L.R. and J.W. conducted in vivo and ex vivo experiments. J.P.H., M.J.E. C.L.S and J.S.L. designed the experiments, analyzed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–22 and Supplementary Tables 1–8 (PDF 8258 kb)
Supplementary Video 1
Maximum intensity projection (MIP) video of the 89Zr-mTf PET image of the Hi-Myc (12 month) mouse shown in Figure 3. The video shows the three-dimensional distribution of 89Zr-mTf at 16 h after administration. The prostate and bladder are visible as spatially resolved masses showing high contrast in the lower abdomen. (WMV 1009 kb)
Rights and permissions
About this article
Cite this article
Holland, J., Evans, M., Rice, S. et al. Annotating MYC status with 89Zr-transferrin imaging. Nat Med 18, 1586–1591 (2012). https://doi.org/10.1038/nm.2935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2935
This article is cited by
-
Ferroptosis landscape in prostate cancer from molecular and metabolic perspective
Cell Death Discovery (2023)
-
Analogues of desferrioxamine B (DFOB) with new properties and new functions generated using precursor-directed biosynthesis
BioMetals (2019)
-
Translational molecular imaging in exocrine pancreatic cancer
European Journal of Nuclear Medicine and Molecular Imaging (2018)
-
Multiplexed imaging for diagnosis and therapy
Nature Biomedical Engineering (2017)
-
Siderophores for molecular imaging applications
Clinical and Translational Imaging (2017)