Abstract
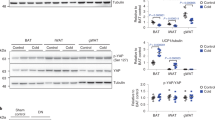
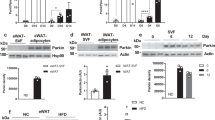
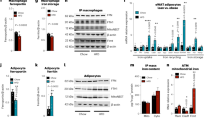
We examined mouse models with altered adipocyte expression of mitoNEET, a protein residing in the mitochondrial outer membrane, to probe its impact on mitochondrial function and subsequent cellular responses. We found that overexpression of mitoNEET enhances lipid uptake and storage, leading to an expansion of the mass of adipose tissue. Despite the resulting massive obesity, benign aspects of adipose tissue expansion prevail, and insulin sensitivity is preserved. Mechanistically, we also found that mitoNEET inhibits mitochondrial iron transport into the matrix and, because iron is a rate-limiting component for electron transport, lowers the rate of β-oxidation. This effect is associated with a lower mitochondrial membrane potential and lower levels of reactive oxygen species–induced damage, along with increased production of adiponectin. Conversely, a reduction in mitoNEET expression enhances mitochondrial respiratory capacity through enhanced iron content in the matrix, ultimately corresponding to less weight gain on a high-fat diet. However, this reduction in mitoNEET expression also causes heightened oxidative stress and glucose intolerance. Thus, manipulation of mitochondrial function by varying mitoNEET expression markedly affects the dynamics of cellular and whole-body lipid homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Muoio, D.M. & Newgard, C.B. Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 75, 367–401 (2006).
Lowell, B.B. & Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 (2005).
Mehta, J.L., Rasouli, N., Sinha, A.K. & Molavi, B. Oxidative stress in diabetes: a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int. J. Biochem. Cell Biol. 38, 794–803 (2006).
Morino, K., Petersen, K.F. & Shulman, G.I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 (suppl. 2), S9–S15 (2006).
Kim, J.Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Scherer, P.E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Holland, W.L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011).
Kelley, D.E., He, J., Menshikova, E.V. & Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950 (2002).
Ritov, V.B. et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54, 8–14 (2005).
Simoneau, J.A., Veerkamp, J.H., Turcotte, L.P. & Kelley, D.E. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 13, 2051–2060 (1999).
Colca, J.R. et al. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metab. 286, E252–E260 (2004).
Paddock, M.L. et al. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc. Natl. Acad. Sci. USA 104, 14342–14347 (2007).
Wiley, S.E., Murphy, A.N., Ross, S.A., van der Geer, P. & Dixon, J.E. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc. Natl. Acad. Sci. USA 104, 5318–5323 (2007).
Wiley, S.E. et al. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J. Biol. Chem. 282, 23745–23749 (2007).
Lin, J., Zhou, T., Ye, K. & Wang, J. Crystal structure of human mitoNEET reveals distinct groups of iron sulfur proteins. Proc. Natl. Acad. Sci. USA 104, 14640–14645 (2007).
Graves, R.A., Tontonoz, P., Platt, K.A., Ross, S.R. & Spiegelman, B.M. Identification of a fat cell enhancer: analysis of requirements for adipose tissue-specific gene expression. J. Cell. Biochem. 49, 219–224 (1992).
Chavez, J.A. & Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 (2012).
Jornayvaz, F.R. & Shulman, G.I. Diacylglycerol activation of protein kinase cepsilon and hepatic insulin resistance. Cell Metab. 15, 574–584 (2012).
Milne, G.L., Sanchez, S.C., Musiek, E.S. & Morrow, J.D. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2, 221–226 (2007).
Langin, D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 53, 482–491 (2006).
Asterholm, I.W. & Scherer, P.E. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am. J. Pathol. 176, 1364–1376 (2010).
Franckhauser, S. et al. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes 51, 624–630 (2002).
Hanson, R.W. & Reshef, L. Glyceroneogenesis revisited. Biochimie 85, 1199–1205 (2003).
Sun, K., Kusminski, C.M. & Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Invest. 121, 2094–2101 (2011).
Zuris, J.A. et al. Facile transfer of [2Fe-2S] clusters from the diabetes drug target mitoNEET to an apo-acceptor protein. Proc. Natl. Acad. Sci. USA 108, 13047–13052 (2011).
Macdonald, V.W., Charache, S. & Hathaway, P.J. Iron deficiency anemia: mitochondrial α-glycerophosphate dehydrogenase in guinea pig skeletal muscle. J. Lab. Clin. Med. 105, 11–18 (1985).
Crooks, D.R., Ghosh, M.C., Haller, R.G., Tong, W.H. & Rouault, T.A. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 115, 860–869 (2010).
Huang, J. et al. Iron overload and diabetes risk: a shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 60, 80–87 (2011).
Mitchell, P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science 206, 1148–1159 (1979).
Rogers, G.W. et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE 6, e21746 (2011).
Rigoulet, M., Yoboue, E.D. & Devin, A. Mitochondrial ROS generation and its regulation: mechanisms involved in H2O2 signaling. Antioxid. Redox Signal. 14, 459–468 (2011).
Wu, M. et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 292, C125–C136 (2007).
Houstis, N., Rosen, E.D. & Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 (2006).
Tarnopolsky, M.A. et al. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1271–R1278 (2007).
Lin, J., Puigserver, P., Donovan, J., Tarr, P. & Spiegelman, B.M. Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1–related transcription coactivator associated with host cell factor. J. Biol. Chem. 277, 1645–1648 (2002).
Bonnard, C. et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 118, 789–800 (2008).
Curtis, J.M. et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes 59, 1132–1142 (2010).
Karelis, A.D. et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 90, 4145–4150 (2005).
McGarry, J.D. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51, 7–18 (2002).
Koh, E.H. et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 56, 2973–2981 (2007).
Frizzell, N. et al. Succination of thiol groups in adipose tissue proteins in diabetes: succination inhibits polymerization and secretion of adiponectin. J. Biol. Chem. 284, 25772–25781 (2009).
Iwabu, M. et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464, 1313–1319 (2010).
Sherratt, H.S. Mitochondria: structure and function. Rev. Neurol. (Paris) 147, 417–430 (1991).
Dey, R. & Moraes, C.T. Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl-x(L) in osteosarcoma cells. J. Biol. Chem. 275, 7087–7094 (2000).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114, 1752–1761 (2004).
Anderson, E.J., Yamazaki, H. & Neufer, P.D. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid–supported respiration. J. Biol. Chem. 282, 31257–31266 (2007).
Anderson, E.J. et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119, 573–581 (2009).
Korshunov, S.S., Skulachev, V.P. & Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 416, 15–18 (1997).
Yu, T., Robotham, J.L. & Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 103, 2653–2658 (2006).
Finck, B.N. et al. A potential link between muscle peroxisome proliferator- activated receptor-α signaling and obesity-related diabetes. Cell Metab. 1, 133–144 (2005).
Nawrocki, A.R. et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 281, 2654–2660 (2006).
Berglund, E.D. et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Invest. 122, 1000–1009 (2012).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25, 402–408 (2001).
Scherer, P.E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Combs, T.P. et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145, 367–383 (2004).
Laplante, M. et al. Tissue-specific postprandial clearance is the major determinant of PPARγ-induced triglyceride lowering in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R57–R66 (2009).
Hultin, M., Carneheim, C., Rosenqvist, K. & Olivecrona, T. Intravenous lipid emulsions: removal mechanisms as compared to chylomicrons. J. Lipid Res. 36, 2174–2184 (1995).
Bligh, E.G. & Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959).
Acknowledgements
We thank P.D. Neufer, G. Schatz and C.B. Newgard for helpful comments and suggestions. We also thank J. Song and J. Xia for technical assistance, in addition to the rest of the Scherer laboratory, R. Unger and D. Clegg for helpful discussions. We also thank P. Blanchard and Y. Deshaies for kindly providing LPL activity measurements, R. Hammer and the University of Texas Southwestern (UTSW) Transgenic Core Facility for the generation of mouse models, as well as the UTSW Metabolic Core Facility for help in the phenotypic characterization of the mice and G. Milne from Vanderbilt University Medical Center for the F2-isoprostane analysis. The authors were supported by US National Institutes of Health grants R01-DK55758, RC1-DK086629 and P01-DK088761 (P.E.S.), K99-DK094973 and an American Heart Association Beginning Grant in Aid 12BGIA8910006 (W.L.H.), R01-DK081842 (D.A.M.), T32-DK091317 (J.A.S.) and Department of Defense Fellowship USAMRMC-BC085909 (J.P.). C.M.K. was supported by a fellowship from the Juvenile Diabetes Foundation (JDRF 3-2008-130).
Author information
Authors and Affiliations
Contributions
C.M.K. conducted all experiments and wrote the manuscript, except the portions indicated below. W.L.H. helped with the 3H-triolein uptake and β-oxidation experiments and performed their analyses. K.S. generated TRE-mitoNEET mice. J.P. helped plan, perform injections and scan fat pads in control AAV and AAV-mitoNEET experiments. S.B.S. generated the AAV-mitoNEET construct. Y.L. performed the DiOC6 ΔΨm experiment using mitoNEET-transfected 3T3-L1 preadipocytes. G.R.A. and C.L. coordinated the generation of shRNA-MitoN knockdown mice. J.A.S. and D.A.M. measured heme iron and provided high-iron-diet–fed and Hfe−/− liver tissues. P.E.S. was involved in the experimental design, experiments, data analysis and data interpretation, in addition to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–4 and Supplementary Methods (PDF 6156 kb)
Rights and permissions
About this article
Cite this article
Kusminski, C., Holland, W., Sun, K. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 18, 1539–1549 (2012). https://doi.org/10.1038/nm.2899
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2899
This article is cited by
-
Mitochondrial CISD1/Cisd accumulation blocks mitophagy and genetic or pharmacological inhibition rescues neurodegenerative phenotypes in Pink1/parkin models
Molecular Neurodegeneration (2024)
-
Rab18 maintains homeostasis of subcutaneous adipose tissue to prevent obesity-induced metabolic disorders
Science China Life Sciences (2024)
-
Coupling of autophagy and the mitochondrial intrinsic apoptosis pathway modulates proteostasis and ageing in Caenorhabditis elegans
Cell Death & Disease (2023)
-
PINK1 and Parkin regulate IP3R-mediated ER calcium release
Nature Communications (2023)
-
Iron, glucose and fat metabolism and obesity: an intertwined relationship
International Journal of Obesity (2023)