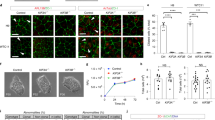
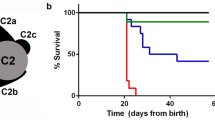
Abstract
Cilia are evolutionarily conserved microtubule-based organelles that are crucial for diverse biological functions, including motility, cell signaling and sensory perception1. In humans, alterations in the formation and function of cilia manifest clinically as ciliopathies, a growing class of pleiotropic genetic disorders2,3,4. Despite the substantial progress that has been made in identifying genes that cause ciliopathies, therapies for these disorders are not yet available to patients. Although mice with a hypomorphic mutation in the intraflagellar transport protein IFT88 (Ift88Tg737Rpw mice, also known as ORPK mice)5 have been well studied, the relevance of IFT88 mutations to human pathology is unknown. We show that a mutation in IFT88 causes a hitherto unknown human ciliopathy. In vivo complementation assays in zebrafish and mIMCD3 cells show the pathogenicity of this newly discovered allele. We further show that ORPK mice are functionally anosmic as a result of the loss of cilia on their olfactory sensory neurons (OSNs). Notably, adenoviral-mediated expression of IFT88 in mature, fully differentiated OSNs of ORPK mice is sufficient to restore ciliary structures and rescue olfactory function. These studies are the first to use in vivo therapeutic treatment to reestablish cilia in a mammalian ciliopathy. More broadly, our studies indicate that gene therapy is a viable option for cellular and functional rescue of the complex ciliary organelle in established differentiated cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosenbaum, J.L. & Witman, G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813–825 (2002).
Sharma, N., Berbari, N.F. & Yoder, B.K. Ciliary dysfunction in developmental abnormalities and diseases. Curr. Top. Dev. Biol. 85, 371–427 (2008).
Lancaster, M.A. & Gleeson, J.G. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 19, 220–229 (2009).
Badano, J.L., Mitsuma, N., Beales, P.L. & Katsanis, N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 7, 125–148 (2006).
Lehman, J.M. et al. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev. Dyn. 237, 1960–1971 (2008).
McEwen, D.P. et al. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc. Natl. Acad. Sci. USA 104, 15917–15922 (2007).
Kulaga, H.M. et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat. Genet. 36, 994–998 (2004).
Tadenev, A.L. et al. Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proc. Natl. Acad. Sci. USA 108, 10320–10325 (2011).
Coon, B.G. et al. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum. Mol. Genet. 21, 335–347 (2012).
Bredrup, C. et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am. J. Hum. Genet. 89, 634–643 (2011).
Valente, E.M. et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat. Genet. 42, 619–625 (2010).
Merrill, A.E. et al. Ciliary abnormalities due to defects in the retrograde transport protein DYNC2H1 in short-rib polydactyly syndrome. Am. J. Hum. Genet. 84, 542–549 (2009).
Walczak-Sztulpa, J. et al. Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am. J. Hum. Genet. 86, 949–956 (2010).
Kozminski, K.G., Johnson, K.A., Forscher, P. & Rosenbaum, J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519–5523 (1993).
Kozminski, K.G., Beech, P.L. & Rosenbaum, J.L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131, 1517–1527 (1995).
Arts, H.H. et al. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J. Med. Genet. 48, 390–395 (2011).
Beales, P.L. et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat. Genet. 39, 727–729 (2007).
Davis, E.E. et al. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet. 43, 189–196 (2011).
Gilissen, C. et al. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am. J. Hum. Genet. 87, 418–423 (2010).
Friedland-Little, J.M. et al. A novel murine allele of Intraflagellar Transport Protein 172 causes a syndrome including VACTERL-like features with hydrocephalus. Hum. Mol. Genet. 20, 3725–3737 (2011).
Pazour, G.J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Willaredt, M.A. et al. A crucial role for primary cilia in cortical morphogenesis. J. Neurosci. 28, 12887–12900 (2008).
Liu, A., Wang, B. & Niswander, L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111 (2005).
Leitch, C.C. et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat. Genet. 40, 443–448 (2008).
Zaghloul, N.A. et al. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc. Natl. Acad. Sci. USA 107, 10602–10607 (2010).
Khanna, H. et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat. Genet. 41, 739–745 (2009).
Acland, G.M. et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 28, 92–95 (2001).
Pawlyk, B.S. et al. Gene replacement therapy rescues photoreceptor degeneration in a murine model of Leber congenital amaurosis lacking RPGRIP. Invest. Ophthalmol. Vis. Sci. 46, 3039–3045 (2005).
Simons, D.L., Boye, S.L., Hauswirth, W.W. & Wu, S.M. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc. Natl. Acad. Sci. USA 108, 6276–6281 (2011).
Bennett, M.K., Kulaga, H.M. & Reed, R.R. Odor-evoked gene regulation and visualization in olfactory receptor neurons. Mol. Cell. Neurosci. 43, 353–362 (2010).
Baker, H., Kawano, T., Margolis, F.L. & Joh, T.H. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J. Neurosci. 3, 69–78 (1983).
Zhao, H. et al. Functional expression of a mammalian odorant receptor. Science 279, 237–242 (1998).
Ivic, L. et al. Adenoviral vector-mediated rescue of the OMP-null phenotype in vivo. Nat. Neurosci. 3, 1113–1120 (2000).
Risser, J.M. & Slotnick, B.M. Nipple attachment and survival in neonatal olfactory bulbectomized rats. Physiol. Behav. 40, 545–549 (1987).
Brunet, L.J., Gold, G.H. & Ngai, J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17, 681–693 (1996).
Varendi, H., Porter, R.H. & Winberg, J. Does the newborn baby find the nipple by smell? Lancet 344, 989–990 (1994).
Sommardahl, C.S., Woychik, R.P., Sweeney, W.E., Avner, E.D. & Wilkinson, J.E. Efficacy of taxol in the orpk mouse model of polycystic kidney disease. Pediatr. Nephrol. 11, 728–733 (1997).
Bisgrove, B.W., Snarr, B.S., Emrazian, A. & Yost, H.J. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev. Biol. 287, 274–288 (2005).
Tsujikawa, M. & Malicki, J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 42, 703–716 (2004).
Malick, L.E. & Wilson, R.B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 50, 265–269 (1975).
Acknowledgements
This work was support by US National Institutes of Health grants R01DC009606 (J.R.M.), F32DC011990 (J.C.M.), R01DC004553, R01DC008295 (R.R.R.), R01DK75996 (B.K.Y.), R01EY021872 (E.E.D.), R01HD04260, R01DK072301 and R01DK075972 (N.K.), by l'Agence National pour la Recherche (ANR) 2010 FOETOCILPATH 1122 01 (T.A.-B.) and by the University of Alabama at Birmingham Hepatorenal Fibrocystic Disease Core Center (DK074083). E.E.D., C.A.J., P.L.B. and N.K. are supported by the European Community's Seventh Framework Programme FP7/2009 under grant agreement 241955, SYSCILIA. N.K. is a Distinguished Jean and George W. Brumley Professor. We thank P. Loget for referring the family for this study, S. Dugan-Rocha, U. Nagaswamy and A. Hawes for assistance with mutational screening and R. Margolskee (Mount Sinai School of Medicine of New York University) for providing antibodies.
Author information
Authors and Affiliations
Contributions
J.C.M., E.E.D., A.J., I.-C.T., S.T., K.S., P.M.J., D.P.M., L.Z. and J.E. performed experiments. T.A.-B., P.L.B. and C.A.J. provided patients for mutational analysis. E.D.G., J.C.M., the NISC Comparative Sequencing Program, A.S., D.M.M. and R.A.G. performed the mutational analysis. J.C.M., P.M.J., D.P.M., E.E.D., N.K., R.R.R., C.L.W., B.K.Y. and J.R.M. designed experiments. All authors contributed insight towards shaping the aims of the project. J.C.M. and J.R.M. wrote the manuscript with the help of comments and suggestions from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8. (PDF 4559 kb)
Supplementary Table 1
Ciliopathy samples with IFT88Met383Lys mutations (DOC 53 kb)
Rights and permissions
About this article
Cite this article
McIntyre, J., Davis, E., Joiner, A. et al. Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nat Med 18, 1423–1428 (2012). https://doi.org/10.1038/nm.2860
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2860