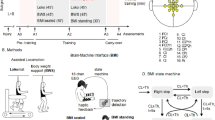
Abstract
Central nervous system (CNS) disorders distinctly impair locomotor pattern generation and balance, but technical limitations prevent independent assessment and rehabilitation of these subfunctions. Here we introduce a versatile robotic interface to evaluate, enable and train pattern generation and balance independently during natural walking behaviors in rats. In evaluation mode, the robotic interface affords detailed assessments of pattern generation and dynamic equilibrium after spinal cord injury (SCI) and stroke. In enabling mode, the robot acts as a propulsive or postural neuroprosthesis that instantly promotes unexpected locomotor capacities including overground walking after complete SCI, stair climbing following partial SCI and precise paw placement shortly after stroke. In training mode, robot-enabled rehabilitation, epidural electrical stimulation and monoamine agonists reestablish weight-supported locomotion, coordinated steering and balance in rats with a paralyzing SCI. This new robotic technology and associated concepts have broad implications for both assessing and restoring motor functions after CNS disorders, both in animals and in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Courtine, G. et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 12, 1333–1342 (2009).
Harkema, S.J. et al. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 77, 797–811 (1997).
Nessler, J.A. et al. A robotic device for studying rodent locomotion after spinal cord injury. IEEE Trans. Neural. Syst. Rehabil. Eng. 13, 497–506 (2005).
Frey, M. et al. A novel mechatronic body weight support system. IEEE Trans. Neural. Syst. Rehabil. Eng. 14, 311–321 (2006).
Winter, D.A., MacKinnon, C.D., Ruder, G.K. & Wieman, C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog. Brain Res. 97, 359–367 (1993).
Warren, W.H. Jr., Kay, B.A., Zosh, W.D., Duchon, A.P. & Sahuc, S. Optic flow is used to control human walking. Nat. Neurosci. 4, 213–216 (2001).
Musselman, K., Brunton, K., Lam, T. & Yang, J. Spinal cord injury functional ambulation profile: a new measure of walking ability. Neurorehabil. Neural Repair 25, 285–293 (2011).
Hidler, J. et al. ZeroG: overground gait and balance training system. J. Rehabil. Res. Dev. 48, 287–298 (2011).
Shetty, D., Fast, A. & Campana, C.A. Ambulatory suspension and rehabilitation apparatus. US patent 7462138 (2008).
Pratt, G.A. et al. Stiffness Isn't Everything. in International Symposium on Experimental Robotics http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.26.3136 (1995).
Vallery, H. et al. Compliant actuation of rehabilitation robots—benefits and limitations of series elastic actuators. IEEE Robot. Autom. Mag. 15, 60–69 (2008).
Harkema, S. et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947 (2011).
Timoszyk, W.K. et al. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 1050, 180–189 (2005).
Zörner, B. et al. Profiling locomotor recovery: comprehensive quantification of impairments after CNS damage in rodents. Nat. Methods 7, 701–708 (2010).
Drew, T., Andujar, J.E., Lajoie, K. & Yakovenko, S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res. Rev. 57, 199–211 (2008).
Courtine, G. et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 14, 69–74 (2008).
Basso, D.M. et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J. Neurotrauma 13, 343–359 (1996).
Musienko, P. et al. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J. Neurosci. 31, 9264–9278 (2011).
Kwakkel, G., Kollen, B.J. & Krebs, H.I. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil. Neural Repair 22, 111–121 (2008).
Duschau-Wicke, A., Caprez, A. & Riener, R. Patient-cooperative control increases active participation of individuals with SCI during robot-aided gait training. J. Neuroeng. Rehabil. 7, 43 (2010).
Peearson, K.G. Generating the walking gait: role of sensory feedback. Prog. Brain Res. 143, 123–129 (2004).
Edgerton, V.R. & Roy, R.R. Robotic training and spinal cord plasticity. Brain Res. Bull. 78, 4–12 (2009).
Wessels, M., Lucas, C., Eriks, I. & de Groot, S. Body weight–supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J. Rehabil. Med. 42, 513–519 (2010).
Reinkensmeyer, D.J. et al. Tools for understanding and optimizing robotic gait training. J. Rehabil. Res. Dev. 43, 657–670 (2006).
Ada, L., Dean, C.M., Vargas, J. & Ennis, S. Mechanically assisted walking with body weight support results in more independent walking than assisted overground walking in non-ambulatory patients early after stroke: a systematic review. J. Physiother. 56, 153–161 (2010).
Colgate, E. & Hogan, N. An analysis of contact instability in terms of passive physical equivalents. Proc. IEEE Int. Conf. Robot. Autom. doi:10.1109/ROBOT.1989.100021 (1989).
Acknowledgements
We would like to acknowledge the excellent technical help provided by J. Heutschi, N. Wenger, M. Hürlimann, Q. Barraud and S. Duis for data collection and care of the rats, as well as A.S. Tafreshi for the design of the user interface to control the robotic system and A. Brunschweiler, A. Rotta and M. Fritschi for the realization of the suspension system. This work was supported by the National Centers of Competence in Research “Neural Plasticity and Repair” and “Robotics” of the Swiss National Science Foundation, the European Research Council (ERC 261247, “Walk Again”), European Community's Seventh Framework Programme (CP-IP 258654, NeuWALK), the Christopher and Dana Reeve Foundation and the Swiss National Science Foundation (subside 310030_130850).
Author information
Authors and Affiliations
Contributions
U.K., H.V., R.R. and G.C. conceived of and designed the robotic interface. P.M., M.L.S. and G.C. performed the surgeries. N.D. and G.C. conceived of the experiments, analyzed the data and prepared the figures with the help of the other authors. N.D., L.F., R.v.d.B. trained the rats and collected the data. G.C. wrote the manuscript, and all the authors contributed to its editing. G.C. supervised all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 8470 kb)
Supplementary Video 1
Design and validation of the robotic interface (MOV 18902 kb)
Supplementary Video 2
Evaluation of optimal support conditions for motor pattern generation in spinal rats (MOV 19058 kb)
Supplementary Video 3
Evaluation of balance control capacities after a cortical stroke (MOV 7764 kb)
Supplementary Video 4
Propulsive neuroprosthetic interface (MOV 9793 kb)
Supplementary Video 5
Postural neuroprosthetic interface to enable precise paw placement after a cortical stroke (MOV 6650 kb)
Supplementary Video 6
Postural neuroprosthetic interface to enable coordinated locomotion in rats with a cervical hemisection (MOV 8024 kb)
Supplementary Video 7
Postural neuroprosthetic interface to enable coordinated locomotion in rats with paralyzing SCI (MOV 9977 kb)
Supplementary Video 8
Robot-enabled locomotor training restores equilibrated steering in rats with paralyzing SCI (MOV 24035 kb)
Rights and permissions
About this article
Cite this article
Dominici, N., Keller, U., Vallery, H. et al. Versatile robotic interface to evaluate, enable and train locomotion and balance after neuromotor disorders. Nat Med 18, 1142–1147 (2012). https://doi.org/10.1038/nm.2845
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2845
This article is cited by
-
Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury
Nature Communications (2021)
-
The development of mature gait patterns in children during walking and running
European Journal of Applied Physiology (2021)
-
Neurorestorative interventions involving bioelectronic implants after spinal cord injury
Bioelectronic Medicine (2019)
-
Multifactorial motor behavior assessment for real-time evaluation of emerging therapeutics to treat neurologic impairments
Scientific Reports (2019)
-
Spinal cord repair: advances in biology and technology
Nature Medicine (2019)