Abstract
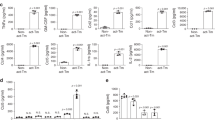
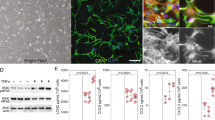
Primary astrocytomas of grade 3 or 4 according to the classification system of the World Health Organization (high-grade astrocytomas or HGAs) are preponderant among adults and are almost invariably fatal despite the use of multimodal therapy. Here we show that the juvenile brain has an endogenous defense mechanism against HGAs. Neural precursor cells (NPCs) migrate to HGAs, reduce glioma expansion and prolong survival time by releasing endovanilloids that activate the vanilloid receptor (transient receptor potential vanilloid subfamily member-1 or TRPV1) on HGA cells. TRPV1 is highly expressed in tumor and weakly expressed in tumor-free brain. TRPV1 stimulation triggers tumor cell death through the branch of the endoplasmic reticulum stress pathway that is controlled by activating transcription factor-3 (ATF3). The antitumorigenic response of NPCs is lost with aging. NPC-mediated tumor suppression can be mimicked in the adult brain by systemic administration of the synthetic vanilloid arvanil, suggesting that TRPV1 agonists have potential as new HGA therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Sanai, N., Alvarez-Buylla, A. & Berger, M.S. Neural stem cells and the origin of gliomas. N. Engl. J. Med. 353, 811–822 (2005).
Ohgaki, H. & Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 109, 93–108 (2005).
Ohgaki, H. & Kleihues, P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 170, 1445–1453 (2007).
Knoth, R. et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5, e8809 (2010).
Leuner, B., Kozorovitskiy, Y., Gross, C.G. & Gould, E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. USA 104, 17169–17173 (2007).
Assanah, M. et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor–expressing retroviruses. J. Neurosci. 26, 6781–6790 (2006).
Assanah, M.C. et al. PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia 1835–1847 (2009).
Glass, R. et al. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J. Neurosci. 25, 2637–2646 (2005).
Walzlein, J.H. et al. The antitumorigenic response of neural precursors depends on subventricular proliferation and age. Stem Cells 26, 2945–2954 (2008).
Aboody, K.S. et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 97, 12846–12851 (2000).
Suzuki, T. et al. Inhibition of glioma cell proliferation by neural stem cell factor. J. Neurooncol. 74, 233–239 (2005).
Staflin, K. et al. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res. 64, 5347–5354 (2004).
Staflin, K., Zuchner, T., Honeth, G., Darabi, A. & Lundberg, C. Identification of proteins involved in neural progenitor cell targeting of gliomas. BMC Cancer 9, 206 (2009).
Starowicz, K., Nigam, S. & Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 114, 13–33 (2007).
Tóth, A., Blumberg, P.M. & Boczan, J. Anandamide and the vanilloid receptor (TRPV1). Vitam. Horm. 81, 389–419 (2009).
Szallasi, A., Cortright, D.N., Blum, C.A. & Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6, 357–372 (2007).
Chirasani, S.R. et al. Bone morphogenetic protein-7 release from endogenous neural precursor cells suppresses the tumourigenicity of stem-like glioblastoma cells. Brain 133, 1961–1972 (2010).
Yamaguchi, M., Saito, H., Suzuki, M. & Mori, K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. Neuroreport 11, 1991–1996 (2000).
Zhao, C., Deng, W. & Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Okano, H., Imai, T. & Okabe, M. Musashi: a translational regulator of cell fate. J. Cell Sci. 115, 1355–1359 (2002).
Cullen, B.R. Enhancing and confirming the specificity of RNAi experiments. Nat. Methods 3, 677–681 (2006).
Lambert, D.M. & Di Marzo, V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic? Curr. Med. Chem. 6, 757–773 (1999).
Ueda, N., Puffenbarger, R.A., Yamamoto, S. & Deutsch, D.G. The fatty acid amide hydrolase (FAAH). Chem. Phys. Lipids 108, 107–121 (2000).
Cravatt, B.F. et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 98, 9371–9376 (2001).
Stürzebecher, A.S. et al. An in vivo tethered toxin approach for the cell-autonomous inactivation of voltage-gated sodium channel currents in nociceptors. J. Physiol. (Lond.) 588, 1695–1707 (2010).
Caterina, M.J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000).
Gallego-Sandín, S., Rodriguez-Garcia, A., Alonso, M.T. & Garcia-Sancho, J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J. Biol. Chem. 284, 32591–32601 (2009).
Hori, O. et al. Role of Herp in the endoplasmic reticulum stress response. Genes Cells 9, 457–469 (2004).
Xu, C., Bailly-Maitre, B. & Reed, J.C. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 115, 2656–2664 (2005).
Kowalczyk, A. et al. The critical role of cyclin D2 in adult neurogenesis. J. Cell Biol. 167, 209–213 (2004).
Unal Cevik, I. & Dalkara, T. Intravenously administered propidium iodide labels necrotic cells in the intact mouse brain after injury. Cell Death Differ. 10, 928–929 (2003).
Melck, D. et al. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem. Biophys. Res. Commun. 262, 275–284 (1999).
Veldhuis, W.B. et al. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J. Neurosci. 23, 4127–4133 (2003).
Fisher, T. et al. Mechanisms operative in the antitumor activity of temozolomide in glioblastoma multiforme. Cancer J. 13, 335–344 (2007).
McConville, P. et al. Magnetic resonance imaging determination of tumor grade and early response to temozolomide in a genetically engineered mouse model of glioma. Clin. Cancer Res. 13, 2897–2904 (2007).
Mrugala, M.M. & Chamberlain, M.C. Mechanisms of disease: temozolomide and glioblastoma–look to the future. Nat. Clin. Pract. Oncol. 5, 476–486 (2008).
Conover, J.C. & Shook, B.A. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2, 149–163 (2011).
Khan, M.A. & Lie, D.C. MicroRNA—a contributor to age-associated neural stem cell dysfunction? Aging 3, 182–183 (2011).
Di Marzo, V., Gobbi, G. & Szallasi, A. Brain TRPV1: a depressing TR(i)P down memory lane? Trends Pharmacol. Sci. 29, 594–600 (2008).
Maccarrone, M., Lorenzon, T., Bari, M., Melino, G. & Finazzi-Agro, A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 275, 31938–31945 (2000).
Izzo, A.A. et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon. J. Mol. Med. 86, 89–98 (2008).
Prevarskaya, N., Zhang, L. & Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 1772, 937–946 (2007).
Bode, A.M. et al. Transient receptor potential type vanilloid 1 suppresses skin carcinogenesis. Cancer Res. 69, 905–913 (2009).
Visnyei, K. et al. A molecular screening approach to identify and characterize inhibitors of glioblastoma stem cells. Mol. Cancer Ther. 10, 1818–1828 (2011).
Morelli, M.B. et al. The Transient Receptor Potential Vanilloid-2 (TRPV2) cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int. J. Cancer published online, doi:10.1002/ijc.27588 (11 April 2012).
Lee, J. et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391–403 (2006).
Grünweller, A. et al. Comparison of different antisense strategies in mammalian cells using locked nucleic acids, 2′-O-methyl RNA, phosphorothioates and small interfering RNA. Nucleic Acids Res. 31, 3185–3193 (2003).
Gurok, U. et al. Gene expression changes in the course of neural progenitor cell differentiation. J. Neurosci. 24, 5982–6002 (2004).
Devane, W.A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992).
Bisogno, T., Maurelli, S., Melck, D., De Petrocellis, L. & Di Marzo, V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 272, 3315–3323 (1997).
Marsicano, G. et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534 (2002).
de Godoy, L.M. et al. Comprehensive mass-spectrometry–based proteome quantification of haploid versus diploid yeast. Nature 455, 1251–1254 (2008).
Kempermann, G., Gast, D., Kronenberg, G., Yamaguchi, M. & Gage, F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130, 391–399 (2003).
Reimer, T.A. et al. Reevaluation of the 22–1-1 antibody and its putative antigen, EBAG9/RCAS1, as a tumor marker. BMC Cancer 5, 47 (2005).
Acknowledgements
We thank G. Gargiulo, O. Daumke and J. Kurreck for discussion of the manuscript, S. Kitajima (Medical Research Institute, Tokyo Medical and Dental University, Tokyo, Japan) for ATF3 constructs and L. Kaczmarek and M. Szymanska (Nencki Institute, Warsaw, Poland) for providing Ccnd2−/− mice. We acknowledge funding from the Helios Clinics (HeFoFö-ID1148) and the US National Institutes of Health (DA-009789 to V.D.M.).
Author information
Authors and Affiliations
Contributions
S.P., R.I., A.L., L.D.P., U.G. and S.R.C. designed and conducted the experiments, and interpreted the data. K.S., J.K., E.S.J.S., P.W., B.P., U.A.N., V.M., B.F.C., S.M., V.D.M., J.-H.W., G.D. and L.C. contributed to manuscript preparation. G.R.L., V.D.M. and H.K. designed the experiments, supervised the project, interpreted the data and contributed to manuscript preparation. M.S. performed brain tumor resections and provided tumor samples. M.S. and R.G. designed and conducted the experiments, supervised the project, interpreted the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2687 kb)
Rights and permissions
About this article
Cite this article
Stock, K., Kumar, J., Synowitz, M. et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat Med 18, 1232–1238 (2012). https://doi.org/10.1038/nm.2827
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2827
This article is cited by
-
Capsaicin alleviates neuronal apoptosis and schizophrenia-like behavioral abnormalities induced by early life stress
Schizophrenia (2023)
-
Identifying the role of transient receptor potential channels (TRPs) in kidney renal clear cell carcinoma and their potential therapeutic significances using genomic and transcriptome analyses
BMC Medical Genomics (2022)
-
Pan-cancer analyses reveal the genetic and pharmacogenomic landscape of transient receptor potential channels
npj Genomic Medicine (2022)
-
Advances in TRP channel drug discovery: from target validation to clinical studies
Nature Reviews Drug Discovery (2022)
-
Ascorbate-induced oxidative stress mediates TRP channel activation and cytotoxicity in human etoposide-sensitive and -resistant retinoblastoma cells
Laboratory Investigation (2021)