Abstract
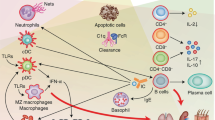
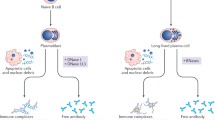
Systemic lupus erythematosus (SLE) is an autoimmune disease that is characterized by the loss of tolerance to nuclear self antigens, the production of pathogenic autoantibodies and damage to multiple organ systems. Over the years, patients with SLE have been managed largely with empiric immunosuppressive therapies, which are associated with substantial toxicities and do not always provide adequate control of the disease. The development of targeted therapies that specifically address disease pathogenesis or progression has lagged, largely because of the complex and heterogeneous nature of the disease, as well as difficulties in designing uniform outcome measures for clinical trials. Recent advances that could improve the treatment of SLE include the identification of genetic variations that influence the risk of developing the disease, an enhanced understanding of innate and adaptive immune activation and regulation of tolerance, dissection of immune cell activation and inflammatory pathways and elucidation of mechanisms and markers of tissue damage. These discoveries, together with improvements in clinical trial design, form a platform from which to launch the development of a new generation of lupus therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lateef, A. & Petri, M. Biologics in the treatment of systemic lupus erythematosus. Curr. Opin. Rheumatol. 22, 504–509 (2010).
Navarra, S.V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Morel, L. et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc. Natl. Acad. Sci. USA 97, 6670–6675 (2000).
Lauwerys, B.R. & Wakeland, E.K. Genetics of lupus nephritis. Lupus 14, 2–12 (2005).
Harley, J.B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 (2008).
Deng, Y. & Tsao, B.P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 6, 683–692 (2010).
Flesher, D.L., Sun, X., Behrens, T.W., Graham, R.R. & Criswell, L.A. Recent advances in the genetics of systemic lupus erythematosus. Expert Rev. Clin. Immunol. 6, 461–479 (2010).
Liu, K. et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J. Clin. Invest. 119, 911–923 (2009).
Sanchez, E. et al. Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann. Rheum. Dis. 70, 1752–1757 (2011).
Cantor, R.M., Lange, K. & Sinsheimer, J.S. Prioritizing GWAS results: a review of statistical methods and recommendations for their application. Am. J. Hum. Genet. 86, 6–22 (2010).
Zhang, J. et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat. Genet. 43, 902–907 (2011).
Rieck, M. et al. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J. Immunol. 179, 4704–4710 (2007).
Taylor, K.E. et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 7, e1001311 (2011).
Askanase, A.D. et al. Use of pharmacogenetics, enzymatic phenotyping, and metabolite monitoring to guide treatment with azathioprine in patients with systemic lupus erythematosus. J. Rheumatol. 36, 89–95 (2009).
Rubtsov, A.V., Rubtsova, K., Kappler, J.W. & Marrack, P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun. Rev. 9, 494–498 (2010).
Smith-Bouvier, D.L. et al. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 205, 1099–1108 (2008).
Ravichandran, K.S. & Lorenz, U. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 7, 964–974 (2007).
Marínez Valle, F., Balada, E., Ordi-Ros, J. & Vilardell-Tarres, M. DNase 1 and systemic lupus erythematosus. Autoimmun. Rev. 7, 359–363 (2008).
Rönnblom, L. & Alm, G.V. The natural interferon-α producing cells in systemic lupus erythematosus. Hum. Immunol. 63, 1181–1193 (2002).
Blanco, P., Palucka, A.K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Bauer, J.W. et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 3, e491 (2006).
Mathian, A., Weinberg, A., Gallegos, M., Banchereau, J. & Koutouzov, S. IFN-α induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J. Immunol. 174, 2499–2506 (2005).
Ramanujam, M. et al. Interferon-α treatment of female (NZW × BXSB)F(1) mice mimics some but not all features associated with the Yaa mutation. Arthritis Rheum. 60, 1096–1101 (2009).
Nacionales, D.C. et al. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 56, 3770–3783 (2007).
Agrawal, H. et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J. Immunol. 183, 6021–6029 (2009).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Thacker, S.G. et al. The detrimental effects of IFN-α on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J. Immunol. 185, 4457–4469 (2010).
Garcia-Romo, G.S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Charles, N., Hardwick, D., Daugas, E., Illei, G.G. & Rivera, J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat. Med. 16, 701–707 (2010).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Boulé, M.W. et al. Toll-like receptor 9–dependent and –independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 199, 1631–1640 (2004).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Tian, J. et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496 (2007).
Gilliet, M., Cao, W. & Liu, Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008).
Rönnblom, L. & Elkon, K.B. Cytokines as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 339–347 (2010).
Leadbetter, E.A. et al. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Berland, R. et al. Toll-like receptor 7–dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity 25, 429–440 (2006).
Christensen, S.R. & Shlomchik, M.J. Regulation of lupus-related autoantibody production and clinical disease by Toll-like receptors. Semin. Immunol. 19, 11–23 (2007).
Christensen, S.R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Fossati, L. et al. The Yaa gene-mediated acceleration of murine lupus: Yaa− T cells from non-autoimmune mice collaborate with Yaa+ B cells to produce lupus autoantibodies in vivo. Eur. J. Immunol. 25, 3412–3417 (1995).
Barbalat, R., Ewald, S.E., Mouchess, M.L. & Barton, G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 29, 185–214 (2011).
Harley, J.B., Harley, I.T., Guthridge, J.M. & James, J.A. The curiously suspicious: a role for Epstein-Barr virus in lupus. Lupus 15, 768–777 (2006).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Ishii, K.J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Zhang, Z. et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 12, 959–965 (2011).
Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131 (2012).
Goodnow, C.C., Vinuesa, C.G., Randall, K.L., Mackay, F. & Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 11, 681–688 (2010).
Arbuckle, M.R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Moulton, V.R. & Tsokos, G.C. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res. Ther. 13, 207 (2011).
Perl, A. et al. T-cell and B-cell signaling biomarkers and treatment targets in lupus. Curr. Opin. Rheumatol. 21, 454–464 (2009).
Crispín, J.C., Kyttaris, V.C., Terhorst, C. & Tsokos, G.C. T cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 6, 317–325 (2010).
Deng, G.M., Liu, L., Bahjat, F.R., Pine, P.R. & Tsokos, G.C. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 62, 2086–2092 (2010).
Ichinose, K., Juang, Y.T., Crispin, J.C., Kis-Toth, K. & Tsokos, G.C. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis Rheum. 63, 523–529 (2011).
Bahjat, F.R. et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum. 58, 1433–1444 (2008).
Linterman, M.A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Simpson, N. et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62, 234–244 (2010).
Odegard, J.M. et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 205, 2873–2886 (2008).
Crispín, J.C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 (2008).
Wardemann, H. & Nussenzweig, M.C. B-cell self-tolerance in humans. Adv. Immunol. 95, 83–110 (2007).
Liu, Z. & Davidson, A. BAFF and selection of autoreactive B cells. Trends Immunol. 32, 388–394 (2011).
Arechiga, A.F. et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J. Immunol. 182, 3343–3347 (2009).
Menard, L. et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 121, 3635–3644 (2011).
Cappione, A. III et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J. Clin. Invest. 115, 3205–3216 (2005).
Gonzalez, S.F. et al. Trafficking of B cell antigen in lymph nodes. Annu. Rev. Immunol. 29, 215–233 (2011).
Kranich, J. et al. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J. Exp. Med. 205, 1293–1302 (2008).
Blair, P.A. et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32, 129–140 (2010).
Campbell, D.J. & Koch, M.A. Treg cells: patrolling a dangerous neighborhood. Nat. Med. 17, 929–930 (2011).
Kim, H.J. et al. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc. Natl. Acad. Sci. USA 108, 2010–2015 (2011).
Brownlie, R.J. et al. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J. Exp. Med. 205, 883–895 (2008).
Kim, S.J. et al. Increased IL-12 inhibits B cell differentiation to germinal center plasma cells and promotes differentiation to short-lived plasmablasts. J. Exp. Med. 205, 2437–2448 (2008).
Herlands, R.A., William, J., Hershberg, U. & Shlomchik, M.J. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur. J. Immunol. 37, 3339–3351 (2007).
Erickson, L.D. et al. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J. Clin. Invest. 109, 613–620 (2002).
Cassese, G. et al. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 31, 2726–2732 (2001).
Tokoyoda, K., Hauser, A.E., Nakayama, T. & Radbruch, A. Organization of immunological memory by bone marrow stroma. Nat. Rev. Immunol. 10, 193–200 (2010).
Merrill, J.T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62, 222–233 (2010).
Benson, M.J. et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180, 3655–3659 (2008).
Jacobi, A.M. et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 62, 201–210 (2010).
Looney, R.J., Anolik, J. & Sanz, I. A perspective on B-cell–targeting therapy for SLE. Mod. Rheumatol. 20, 1–10 (2010).
Aringer, M. et al. Current state of evidence on “off label” therapeutic options for systemic lupus erythematosus, including biological immunosuppressive agents, in Germany, Austria, and Switzerland—a consensus report. Lupus 21, 386–401 (2012).
Hahn, B.H. Targeted therapies in systemic lupus erythematosus: successes, failures and future. Ann. Rheum. Dis. 70 (suppl. 1), i64–i66 (2011).
de Laat, B., Mertens, K. & de Groot, P.G. Mechanisms of disease: antiphospholipid antibodies—from clinical association to pathologic mechanism. Nat. Clin. Pract. Rheumatol. 4, 192–199 (2008).
Lauvsnes, M.B. & Omdal, R. Systemic lupus erythematosus, the brain, and anti-NR2 antibodies. J. Neurol. 259, 622–629 (2012).
Faust, T.W. et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc. Natl. Acad. Sci. USA 107, 18569–18574 (2010).
DeGiorgio, L.A. et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 7, 1189–1193 (2001).
Matus, S. et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J. Exp. Med. 204, 3221–3234 (2007).
Kowal, C. et al. Cognition and immunity; antibody impairs memory. Immunity 21, 179–188 (2004).
Turnberg, D. & Cook, H.T. Complement and glomerulonephritis: new insights. Curr. Opin. Nephrol. Hypertens. 14, 223–228 (2005).
Bergtold, A., Gavhane, A., D'Agati, V., Madaio, M. & Clynes, R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J. Immunol. 177, 7287–7295 (2006).
Anders, H.J. & Schlondorff, D. Toll-like receptors: emerging concepts in kidney disease. Curr. Opin. Nephrol. Hypertens. 16, 177–183 (2007).
Manderson, A.P., Botto, M. & Walport, M.J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 22, 431–456 (2004).
Woodruff, T.M., Nandakumar, K.S. & Tedesco, F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 48, 1631–1642 (2011).
Vielhauer, V., Anders, H.J. & Schlondorff, D. Chemokines and chemokine receptors as therapeutic targets in lupus nephritis. Semin. Nephrol. 27, 81–97 (2007).
Kitching, A.R. & Holdsworth, S.R. The emergence of TH17 cells as effectors of renal injury. J. Am. Soc. Nephrol. 22, 235–238 (2011).
Ernandez, T. & Mayadas, T.N. Immunoregulatory role of TNFα in inflammatory kidney diseases. Kidney Int. 76, 262–276 (2009).
Bethunaickan, R. et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J. Immunol. 186, 4994–5003 (2011).
Hill, G.S. et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 59, 304–316 (2001).
Schlondorff, D.O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 74, 860–866 (2008).
Deelman, L. & Sharma, K. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr. Opin. Nephrol. Hypertens. 18, 85–90 (2009).
Alarcón, G.S. et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 3, e396 (2006).
Esdaile, J.M. et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 44, 2331–2337 (2001).
Symmons, D.P. & Gabriel, S.E. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat. Rev. Rheumatol. 7, 399–408 (2011).
Narshi, C.B., Giles, I.P. & Rahman, A. The endothelium: an interface between autoimmunity and atherosclerosis in systemic lupus erythematosus? Lupus 20, 5–13 (2011).
Lopez, L.R. et al. Oxidized low-density lipoprotein and β2-glycoprotein I in patients with systemic lupus erythematosus and increased carotid intima-media thickness: implications in autoimmune-mediated atherosclerosis. Lupus 15, 80–86 (2006).
Matsuura, E., Kobayashi, K., Hurley, B.L. & Lopez, L.R. Atherogenic oxidized low-density lipoprotein/β2-glycoprotein I (oxLDL/β2GPI) complexes in patients with systemic lupus erythematosus and antiphospholipid syndrome. Lupus 15, 478–483 (2006).
Skaggs, B.J., Hahn, B.H., Sahakian, L., Grossman, J. & McMahon, M. Dysfunctional, pro-inflammatory HDL directly upregulates monocyte PDGFRβ, chemotaxis and TNFα production. Clin. Immunol. 137, 147–156 (2010).
McMahon, M. et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheum. 60, 2428–2437 (2009).
Schanberg, L.E. et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 64, 285–296 (2012).
Petri, M.A., Kiani, A.N., Post, W., Christopher-Stine, L. & Magder, L.S. Lupus Atherosclerosis Prevention Study (LAPS). Ann. Rheum. Dis. 70, 760–765 (2011).
Ceribelli, A., Yao, B., Dominguez-Gutierrez, P.R. & Chan, E.K. Lupus T cells switched on by DNA hypomethylation via microRNA? Arthritis Rheum. 63, 1177–1181 (2011).
Pan, Y. & Sawalha, A.H. Epigenetic regulation and the pathogenesis of systemic lupus erythematosus. Transl. Res. 153, 4–10 (2009).
Dai, R. & Ahmed, S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 157, 163–179 (2011).
Ceribelli, A. et al. MicroRNAs in systemic rheumatic diseases. Arthritis Res. Ther. 13, 229 (2011).
Geuking, M.B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Illei, G.G. et al. Current state and future directions of autologous hematopoietic stem cell transplantation in systemic lupus erythematosus. Ann. Rheum. Dis. 70, 2071–2074 (2011).
Choi, E.W. et al. Reversal of serological, immunological and histological dysfunction in systemic lupus erythematosus mice by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum. 64, 243–253 (2012).
Liang, J. et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann. Rheum. Dis. 69, 1423–1429 (2010).
Mok, C.C. Biomarkers for lupus nephritis: a critical appraisal. J. Biomed. Biotechnol. 2010, 638413 (2010).
Bauer, J.W. et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 60, 3098–3107 (2009).
Chaussabel, D. et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity 29, 150–164 (2008).
Hinze, C.H. et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 60, 2772–2781 (2009).
Rovin, B.H. et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J. Am. Soc. Nephrol. 16, 467–473 (2005).
Rubinstein, T. et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology (Oxford) 49, 960–971 (2010).
Guiducci, C. et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465, 937–941 (2010).
Yuan, W., DiMartino, S.J., Redecha, P.B., Ivashkiv, L.B. & Salmon, J.E. Systemic lupus erythematosus monocytes are less responsive to interleukin-10 in the presence of immune complexes. Arthritis Rheum. 63, 212–218 (2011).
Ramanujam, M. & Davidson, A. Targeting of the immune system in systemic lupus erythematosus. Expert Rev. Mol. Med. 10, e2 (2008).
Wofsy, D.S., Shropshire, S.M., Hillson, J.L. & Diamond, B. Abatacept for lupus nephritis: alternative outcome measures support opposing interpretations of data From a multicenter, randomized, double-blind, placebo-controlled phase II/III study. ACR presentation, number 2474 (8 November 2011).
Lenert, P.S. Classification, mechanisms of action, and therapeutic applications of inhibitory oligonucleotides for Toll-like receptors (TLR) 7 and 9. Mediators Inflamm. 2010, 986596 (2010).
Kyttaris, V.C. & Tsokos, G.C. Targeting lymphocyte signaling pathways as a therapeutic approach to systemic lupus erythematosus. Curr. Opin. Rheumatol. 23, 449–453 (2011).
Schiffer, L. et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J. Immunol. 171, 489–497 (2003).
Daikh, D.I. & Wofsy, D. Cutting edge: reversal of murine lupus nephritis with CTLA4Ig and cyclophosphamide. J. Immunol. 166, 2913–2916 (2001).
Ng, K.P. et al. B cell depletion therapy in systemic lupus erythematosus: long-term follow-up and predictors of response. Ann. Rheum. Dis. 66, 1259–1262 (2007).
Bloom, O. et al. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. Proc. Natl. Acad. Sci. USA 108, 10255–10259 (2011).
Clynes, R., Dumitru, C. & Ravetch, J.V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052–1054 (1998).
Gambaro, G. & Kong, N.C. Glycosaminoglycan treatment in glomerulonephritis? An interesting option to investigate. J. Nephrol. 23, 244–252 (2010).
Nguyen, T.Q. & Goldschmeding, R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharm. Res. 25, 2416–2426 (2008).
Renner, B. et al. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int. 80, 165–173 (2011).
Tse, K.C. et al. Angiotensin inhibition or blockade for the treatment of patients with quiescent lupus nephritis and persistent proteinuria. Lupus 14, 947–952 (2005).
McMahon, M., Hahn, B.H. & Skaggs, B.J. Systemic lupus erythematosus and cardiovascular disease: prediction and potential for therapeutic intervention. Expert Rev. Clin. Immunol. 7, 227–241 (2011).
Stohl, W. et al. Belimumab reduces autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. published online doi:10.1002/art.34400 (24 January 2012).
Liu, Z. & Davidson, A. BAFF inhibition: a new class of drugs for the treatment of autoimmunity. Exp. Cell Res. 317, 1270–1277 (2011).
Mackay, F. & Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 (2009).
Stohl, W. et al. Belimumab reduces autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. published online doi:10.1002/art.34400 (24 January 2012).
Acknowledgements
This work was supported by US National Institutes of Health grants R01 DK085241-01, R01 AI083901 and R21 AR057930. The authors thank T. Rothstein and A. Boneparth for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Liu, Z., Davidson, A. Taming lupus—a new understanding of pathogenesis is leading to clinical advances. Nat Med 18, 871–882 (2012). https://doi.org/10.1038/nm.2752
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2752
This article is cited by
-
Transcriptional profiling upon T cell stimulation reveals down-regulation of inflammatory pathways in T and B cells in SLE versus Sjögren’s syndrome
npj Systems Biology and Applications (2023)
-
Formyl peptide receptor 2 as a potential therapeutic target for inflammatory bowel disease
Acta Pharmacologica Sinica (2023)
-
A biological and a mathematical model of SLE treated by mesenchymal stem cells covering all the stages of the disease
Theory in Biosciences (2023)
-
Effect of add-on hydroxychloroquine therapy on serum proinflammatory cytokine levels in patients with systemic lupus erythematosus
Scientific Reports (2022)
-
Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach
Nature Reviews Rheumatology (2022)