Abstract
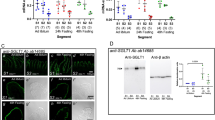
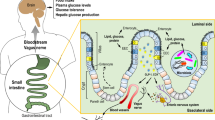
Gastrointestinal bypass surgeries restore metabolic homeostasis in patients with type 2 diabetes and obesity1, but the underlying mechanisms remain elusive. Duodenal-jejunal bypass surgery (DJB), an experimental surgical technique that excludes the duodenum and proximal jejunum from nutrient transit1,2, lowers glucose concentrations in nonobese type 2 diabetic rats2,3,4,5. Given that DJB redirects and enhances nutrient flow into the jejunum and that jejunal nutrient sensing affects feeding6,7, the repositioned jejunum after DJB represents a junction at which nutrients could regulate glucose homeostasis. Here we found that intrajejunal nutrient administration lowered endogenous glucose production in normal rats through a gut-brain-liver network in the presence of basal plasma insulin concentrations. Inhibition of jejunal glucose uptake or formation of long chain fatty acyl-coA negated the metabolic effects of glucose or lipid, respectively, in normal rats, and altered the rapid (2 d) glucose-lowering effect induced by DJB in streptozotocin (STZ)-induced uncontrolled diabetic rats during refeeding. Lastly, in insulin-deficient autoimmune type 1 diabetic rats and STZ-induced diabetic rats, DJB lowered glucose concentrations in 2 d independently of changes in plasma insulin concentrations, food intake and body weight. These data unveil a glucoregulatory role of jejunal nutrient sensing and its relevance in the early improvement of glycemic control after DJB in rat models of uncontrolled diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rubino, F., Schauer, P.R., Kaplan, L.M. & Cummings, D.E. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu. Rev. Med. 61, 393–411 (2010).
Rubino, F. & Marescaux, J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann. Surg. 239, 1–11 (2004).
Wang, T.T. et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann. Surg. 247, 968–975 (2008).
Pacheco, D. et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am. J. Surg. 194, 221–224 (2007).
Kindel, T.L., Yoder, S.M., Seeley, R.J., D'Alessio, D.A. & Tso, P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J. Gastrointest. Surg. 13, 1762–1772 (2009).
Drewe, J., Gadient, A., Rovati, L.C. & Beglinger, C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology 102, 1654–1659 (1992).
Ogawa, N. et al. The vagal afferent pathway does not play a major role in the induction of satiety by intestinal fatty acid in rats. Neurosci. Lett. 433, 38–42 (2008).
Cohen, R.V. et al. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg. Obes. Relat. Dis. 3, 195–197 (2007).
Cohen, R.V. et al. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg. Obes. Relat. Dis. published online, doi:10.1016/j.soard.2012.01.017 (2 February 2012).
Badman, M.K. & Flier, J.S. The gut and energy balance: visceral allies in the obesity wars. Science 307, 1909–1914 (2005).
Cummings, D.E. & Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Invest. 117, 13–23 (2007).
Murphy, K.G. & Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 444, 854–859 (2006).
Coll, A.P., Farooqi, I.S. & O'Rahilly, S. The hormonal control of food intake. Cell 129, 251–262 (2007).
Lam, T.K. Neuronal regulation of homeostasis by nutrient sensing. Nat. Med. 16, 392–395 (2010).
Greenberg, D., Smith, G.P. & Gibbs, J. Intraduodenal infusions of fats elicit satiety in sham-feeding rats. Am. J. Physiol. 259, R110–R118 (1990).
Matzinger, D. et al. The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 46, 688–693 (2000).
Wang, P.Y. et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature 452, 1012–1016 (2008).
Cheung, G.W., Kokorovic, A., Lam, C.K., Chari, M. & Lam, T.K. Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metab. 10, 99–109 (2009).
Lal, S., Kirkup, A.J., Brunsden, A.M., Thompson, D.G. & Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G907–G915 (2001).
Randich, A. et al. Jejunal administration of linoleic acid increases activity of neurons in the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R166–R173 (2004).
Ehrenkranz, J.R., Lewis, N.G., Kahn, C.R. & Roth, J. Phlorizin: a review. Diabetes Metab. Res. Rev. 21, 31–38 (2005).
Mordes, J.P., Bortell, R., Blankenhorn, E.P., Rossini, A.A. & Greiner, D.L. Rat models of type 1 diabetes: genetics, environment and autoimmunity. ILAR J. 45, 278–291 (2004).
Cohen, R., Pinheiro, J.S., Correa, J.L. & Schiavon, C.A. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg. Obes. Relat Dis. 2, 401–404 (2006).
Kim, Z. & Hur, K.Y. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J. Surg. 35, 631–636 (2011).
Thaler, J.P. & Cummings, D.E. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology 150, 2518–2525 (2009).
Rubino, F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 31 (suppl. 2), S290–S296 (2008).
Cox, J.E., Kelm, G.R., Meller, S.T., Spraggins, D.S. & Randich, A. Truncal and hepatic vagotomy reduce suppression of feeding by jejunal lipid infusions. Physiol. Behav. 81, 29–36 (2004).
Troy, S. et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 8, 201–211 (2008).
Chambers, A.P. et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141, 950–958 (2011).
Patti, M.E. et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 17, 1671–1677 (2009).
Watkins, J.B. III & Dykstra, T.P. Alterations in biliary excretory function by streptozotocin-induced diabetes. Drug Metab. Dispos. 15, 177–183 (1987).
Breen, D.M. et al. Duodenal PKC-δ and cholecystokinin signaling axis regulates glucose production. Diabetes 60, 3148–3153 (2011).
Kokorovic, A. et al. Duodenal mucosal protein kinase C-δ regulates glucose production in rats. Gastroenterology 141, 1720–1727 (2011).
Acknowledgements
We are extremely grateful to P.Y.T. Wang for excellent technical assistance. This work was supported by a research grant to T.K.T.L. from the Canadian Institutes of Health Research (MOP-82701). D.M.B. is supported by a post-doctoral fellowship from the University Health Network and the Banting and Best Diabetes Centre (BBDC), University of Toronto. B.A.R. is supported by a BBDC graduate scholarship. A.K. and G.W.C.C. were supported by Canadian Institutes of Health Research and BBDC graduate scholarships. T.K.T.L. holds the John Kitson McIvor (1915–1942) Endowed Chair in Diabetes Research and the Canada Research Chair in Obesity at the Toronto General Research Institute and the University of Toronto.
Author information
Authors and Affiliations
Contributions
D.M.B. conducted and designed experiments, performed data analyses and wrote the manuscript; B.A.R., A.K. and G.W.C.C. assisted in experiments; R.W. assisted in setting up the DJB surgical procedure; and T.K.T.L. supervised the project, designed experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–3 and Supplementary Methods (PDF 2324 kb)
Rights and permissions
About this article
Cite this article
Breen, D., Rasmussen, B., Kokorovic, A. et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med 18, 950–955 (2012). https://doi.org/10.1038/nm.2745
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2745
This article is cited by
-
The Significance of Bile in the Biliopancreatic Limb on Metabolic Improvement After Duodenal-Jejunal Bypass
Obesity Surgery (2024)
-
Effect of duodenal-jejunal bypass on diabetes in the early postoperative period
Scientific Reports (2023)
-
Current status of metabolic surgery in patients with type I diabetes mellitus and obesity: a nationwide multicenter study
Langenbeck's Archives of Surgery (2023)
-
Temporal Relationship Between Insulin Resistance and Lipid Accumulation After Bariatric Surgery: a Multicenter Cohort Study
Obesity Surgery (2023)
-
Choice of Bariatric Surgery in Patients with Obesity and Type 1 Diabetes Mellitus? an Up-to-Date Systematic Review
Obesity Surgery (2022)