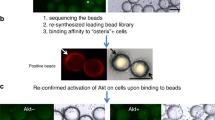
Abstract
Aging reduces the number of mesenchymal stem cells (MSCs) that can differentiate into osteoblasts in the bone marrow, which leads to impairment of osteogenesis. However, if MSCs could be directed toward osteogenic differentiation, they could be a viable therapeutic option for bone regeneration. We have developed a method to direct MSCs to the bone surface by attaching a synthetic high-affinity and specific peptidomimetic ligand (LLP2A) against integrin α4β1 on the MSC surface to a bisphosphonate (alendronate, Ale) that has a high affinity for bone. LLP2A-Ale induced MSC migration and osteogenic differentiation in vitro. A single intravenous injection of LLP2A-Ale increased trabecular bone formation and bone mass in both xenotransplantation studies and in immunocompetent mice. Additionally, LLP2A-Ale prevented trabecular bone loss after peak bone acquisition was achieved or as a result of estrogen deficiency. These results provide proof of principle that LLP2A-Ale can direct MSCs to the bone to form new bone and increase bone strength.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stolzing, A., Jones, E., McGonagle, D. & Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 129, 163–173 (2008).
Katsara, O. et al. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow–derived mesenchymal stem cells. Stem Cells Dev. 20, 1549–1561 (2011).
Bonyadi, M. et al. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. USA 100, 5840–5845 (2003).
Zhang, Y. et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials 31, 5312–5324 (2010).
Pedram, M.S. et al. Transplantation of a combination of autologous neural differentiated and undifferentiated mesenchymal stem cells into injured spinal cord of rats. Spinal Cord 48, 457–463 (2010).
Li, H., Yan, F., Lei, L., Li, Y. & Xiao, Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs 190, 94–101 (2009).
Gutwald, R. et al. Mesenchymal stem cells and inorganic bovine bone mineral in sinus augmentation: comparison with augmentation by autologous bone in adult sheep. Br. J. Oral Maxillofac. Surg. 48, 285–290 (2010).
Vertenten, G. et al. Evaluation of an injectable, photopolymerizable, and three-dimensional scaffold based on methacrylate-endcapped poly(D,L-lactide-co-epsilon-caprolactone) combined with autologous mesenchymal stem cells in a goat tibial unicortical defect model. Tissue Eng. Part A 15, 1501–1511 (2009).
Halleux, C., Sottile, V., Gasser, J.A. & Seuwen, K. Multi-lineage potential of human mesenchymal stem cells following clonal expansion. J. Musculoskelet. Neuronal Interact. 2, 71–76 (2001).
Longobardi, L. et al. Subcellular localization of IRS-1 in IGF-I–mediated chondrogenic proliferation, differentiation and hypertrophy of bone marrow mesenchymal stem cells. Growth Factors 27, 309–320 (2009).
Chapel, A. et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 5, 1028–1038 (2003).
Granero-Moltó, F. et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27, 1887–1898 (2009).
Gao, J., Dennis, J.E., Muzic, R.F., Lundberg, M. & Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169, 12–20 (2001).
Meyerrose, T.E. et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells 25, 220–227 (2007).
Cho, S.W. et al. Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice. Mol. Ther. 17, 1979–1987 (2009).
Jürg, A. Gasser, L.C.C., Kamibayashi, L.K. Intravenously administered mesenchymal OVX-induced bone loss in distribution of labeled MSCs. J. Bone Miner. Res. 14, 1 (1999).
Granero-Moltó, F. et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27, 1887–1898 (2009).
Owen, M. & Friedenstein, A.J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found. Symp. 136, 42–60 (1988).
Bruder, S.P., Fink, D.J. & Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J. Cell. Biochem. 56, 283–294 (1994).
Muraglia, A., Cancedda, R. & Quarto, R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J. Cell Sci. 113, 1161–1166 (2000).
Adams, G.B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006).
Chen, X.D., Dusevich, V., Feng, J.Q., Manolagas, S.C. & Jilka, R.L. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner. Res. 22, 1943–1956 (2007).
Grzesik, W.J. & Robey, P.G. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 9, 487–496 (1994).
Vukicevic, S., Luyten, F.P., Kleinman, H.K. & Reddi, A.H. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell 63, 437–445 (1990).
Gronthos, S., Simmons, P.J., Graves, S.E. & Robey, P.G. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone 28, 174–181 (2001).
Gronthos, S., Stewart, K., Graves, S.E., Hay, S. & Simmons, P.J. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 12, 1189–1197 (1997).
Brooke, G., Tong, H., Levesque, J.P. & Atkinson, K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 17, 929–940 (2008).
Hamidouche, Z. et al. Priming integrin α5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA 106, 18587–18591 (2009).
Mukherjee, S. et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J. Clin. Invest. 118, 491–504 (2008).
Peng, L. et al. Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat. Chem. Biol. 2, 381–389 (2006).
Luo, J. et al. Rainbow beads: a color coding method to facilitate high-throughput screening and optimization of one-bead one-compound combinatorial libraries. J. Comb. Chem. 10, 599–604 (2008).
Yao, W. et al. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PLoS ONE 5, e11410 (2010).
Cao, J., Venton, L., Sakata, T. & Halloran, B.P. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J. Bone Miner. Res. 18, 270–277 (2003).
Sato, M. et al. Abnormal bone architecture and biomechanical properties with near-lifetime treatment of rats with PTH. Endocrinology 143, 3230–3242 (2002).
Dao, M.A., Taylor, N. & Nolta, J.A. Reduction in levels of the cyclin-dependent kinase inhibitor p27(kip-1) coupled with transforming growth factor beta neutralization induces cell-cycle entry and increases retroviral transduction of primitive human hematopoietic cells. Proc. Natl. Acad. Sci. USA 95, 13006–13011 (1998).
Seeman, E. Periosteal bone formation—a neglected determinant of bone strength. N. Engl. J. Med. 349, 320–323 (2003).
Kumar, S. & Ponnazhagan, S. Bone homing of mesenchymal stem cells by ectopic α4 integrin expression. FASEB J. 21, 3917–3927 (2007).
Parfitt, A.M. et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2, 595–610 (1987).
Yao, W. et al. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. 58, 3485–3497 (2008).
Yao, W. et al. Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. J. Bone Miner. Res. 25, 190–199 (2010).
Acknowledgements
The mouse MSCs used in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from National Center for Research Resources of the US National Institutes of Health grant P40RR017447. This work was funded by the US National Institutes of Health grants NIAMS- 5R21AR057515 (to W.Y.), NIAM- R01AR043052 (to N.E.L.) and 1K12HD05195801 and was co-funded by the US National Institute of Child Health and Human Development (NICHD), the Office of Research on Women's Health (ORWH), the Office of Dietary Supplements (ODS) and the US National Institute of Aging (NIA).
Author information
Authors and Affiliations
Contributions
W.Y., N.E.L. and K.S.L. designed the study. M.G. and W.Y. performed the animal study, collected data from the cell cultures, biochemistry, microCT and bone histomorphometry and analyzed all the data. R.L. and L.M. designed and synthesized the compound LLP2A-Ale. R.L. participated in the synthesis of LLP2A-Ale, and K.L. synthesized the LLP2A-Ale. J.N. and P.Z. performed human MSC cultures and designed the experiments using the NOD/SCID/MPSVII mice. J.J. and M.S. performed immunohistochemistry and helped with the animal studies. B. Panganiban and R.O.R. performed biomechanical testing and analyzed data. All authors edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 7871 kb)
Rights and permissions
About this article
Cite this article
Guan, M., Yao, W., Liu, R. et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18, 456–462 (2012). https://doi.org/10.1038/nm.2665
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2665
This article is cited by
-
Targeting strategies for bone diseases: signaling pathways and clinical studies
Signal Transduction and Targeted Therapy (2023)
-
Impaired function of skeletal stem cells derived from growth plates in ovariectomized mice
Journal of Bone and Mineral Metabolism (2023)
-
Shikonin promotes rat periodontal bone defect repair and osteogenic differentiation of BMSCs by p38 MAPK pathway
Odontology (2023)
-
Long non-coding RNA SNHG5 promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the miR-212-3p/GDF5/SMAD pathway
Stem Cell Research & Therapy (2022)
-
CHD7 regulates bone-fat balance by suppressing PPAR-γ signaling
Nature Communications (2022)