Abstract
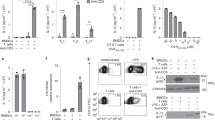
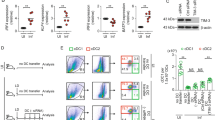
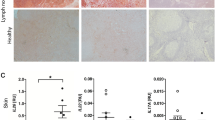
It is unclear whether the ability of the innate immune system to recognize distinct ligands from a single microbial pathogen via multiple pattern recognition receptors (PRRs) triggers common pathways or differentially triggers specific host responses. In the human mycobacterial infection leprosy, we found that activation of monocytes via nucleotide-binding oligomerization domain-containing protein 2 (NOD2) by its ligand muramyl dipeptide, as compared to activation via heterodimeric Toll-like receptor 2 and Toll-like receptor 1 (TLR2/1) by triacylated lipopeptide, preferentially induced differentiation into dendritic cells (DCs), which was dependent on a previously unknown interleukin-32 (IL-32)-dependent mechanism. Notably, IL-32 was sufficient to induce monocytes to rapidly differentiate into DCs, which were more efficient than granulocyte-macrophage colony–stimulating factor (GM-CSF)-derived DCs in presenting antigen to major histocompatibility complex (MHC) class I–restricted CD8+ T cells. Expression of NOD2 and IL-32 and the frequency of CD1b+ DCs at the site of leprosy infection correlated with the clinical presentation; they were greater in patients with limited as compared to progressive disease. The addition of recombinant IL-32 restored NOD2-induced DC differentiation in patients with the progressive form of leprosy. In conclusion, the NOD2 ligand–induced, IL-32–dependent DC differentiation pathway contributes a key and specific mechanism for host defense against microbial infection in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C.A.J. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Brightbill, H.D. et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285, 732–736 (1999).
Ferwerda, G. et al. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 1, 279–285 (2005).
Bloom, B.R. Learning from leprosy: A perspective on immunology and the third world. J. Immunol. 137, i–x (1986).
Ridley, D.S. & Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34, 255–273 (1966).
Yamamura, M. et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254, 277–279 (1991).
Salgame, P. et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254, 279–282 (1991).
Sieling, P.A. et al. CD1 expression by dendritic cells in human leprosy lesions: Correlation with effective host immunity. J. Immunol. 162, 1851–1858 (1999).
Takeuchi, O. et al. Role of TLR1 in mediating immune response to microbial lipoproteins. J. Immunol. 169, 10–14 (2002).
Krutzik, S.R. et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 9, 525–532 (2003).
Kang, T.J. & Chae, G.T. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 31, 53–58 (2001).
Kang, T.J., Lee, S.B. & Chae, G.T. A polymorphism in the Toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine 20, 56–62 (2002).
Bochud, P.Y., Hawn, T.R. & Aderem, A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 170, 3451–3454 (2003).
Schröder, N.W. et al. High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J. Mol. Med. 81, 368–372 (2003).
Kang, T.J., Yeum, C.E., Kim, B.C., You, E.Y. & Chae, G.T. Differential production of interleukin-10 and interleukin-12 in mononuclear cells from leprosy patients with a Toll-like receptor 2 mutation. Immunology 112, 674–680 (2004).
Malhotra, D., Relhan, V., Reddy, B.S. & Bamezai, R. TLR2 Arg677Trp polymorphism in leprosy: revisited. Hum. Genet. 116, 413–415 (2005).
Johnson, C.M. et al. Cutting edge: A common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J. Immunol. 178, 7520–7524 (2007).
Misch, E.A. et al. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl. Trop. Dis. 2, e231 (2008).
Schuring, R.P. et al. Polymorphism N248S in the human Toll-like receptor 1 gene is related to leprosy and leprosy reactions. J. Infect. Dis. 199, 1816–1819 (2009).
Yang, Y. et al. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J. Biol. Chem. 282, 36223–36229 (2007).
Girardin, S.E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003).
Zhang, F.R. et al. Genomewide association study of leprosy. N. Engl. J. Med. 361, 2609–2618 (2009).
Berrington, W.R. et al. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J. Infect. Dis. 201, 1422–1435 (2010).
Krutzik, S.R. et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11, 653–660 (2005).
Niazi, K.R. et al. Activation of human CD4+ T cells by targeting MHC class II epitopes to endosomal compartments using human CD1 tail sequences. Immunology 122, 522–531 (2007).
Netea, M.G. et al. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc. Natl. Acad. Sci. USA 105, 3515–3520 (2008).
Netea, M.G. et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-γ–dependent mechanism. PLoS Med. 3, e277 (2006).
Bai, X. et al. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 184, 3830–3840 (2010).
Nold, M.F. et al. Endogenous IL-32 controls cytokine and HIV-1 production. J. Immunol. 181, 557–565 (2008).
Li, W. et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS ONE 3, e1985 (2008).
Joosten, L.A. et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 103, 3298–3303 (2006).
Shioya, M. et al. Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clin. Exp. Immunol. 149, 480–486 (2007).
Marcondes, A.M. et al. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc. Natl. Acad. Sci. USA 105, 2865–2870 (2008).
Lee, D.J. et al. LILRA2 activation inhibits dendritic cell differentiation and antigen presentation to T cells. J. Immunol. 179, 8128–8136 (2007).
Bleharski, J.R. et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science 301, 1527–1530 (2003).
Montoya, D. et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe 6, 343–353 (2009).
Sieling, P.A. et al. CD1-restricted T cell recognition of microbial lipoglycans. Science 269, 227–230 (1995).
Sieling, P.A. et al. Immunosuppressive roles for interleukin-10 and interleukin-4 in human infection: in vitro modulation of T cell responses in leprosy. J. Immunol. 150, 5501–5510 (1993).
Krutzik, S.R. et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D–dependent antimicrobial pathway. J. Immunol. 181, 7115–7120 (2008).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony–stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor αa. J. Exp. Med. 179, 1109–1118 (1994).
Silva, C.L., Palacios, A., Colston, M.J. & Lowrie, D.B. Mycobacterium leprae 65hsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microb. Pathog. 12, 27–38 (1992).
Modlin, R.L., Hofman, F.M., Taylor, C.R. & Rea, T.H. T lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8, 182–189 (1983).
Jung, M.Y., Son, M.H., Kim, S.H., Cho, D. & Kim, T.S. IL-32γ induces the maturation of dendritic cells with TH1- and TH17-polarizing ability through enhanced IL-12 and IL-6 production. J. Immunol. 186, 6848–6859 (2011).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am. J. Med. Sci. 105, 487–511 (1893).
Morales, A., Eidinger, D. & Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Morton, D., Eilber, F.R., Malmgren, R.A. & Wood, W.C. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery 68, 158–163 (1970).
Adam, A., Ciorbaru, R., Ellouz, F., Petit, J.F. & Lederer, E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem. Biophys. Res. Commun. 56, 561–567 (1974).
Ellouz, F., Adam, A., Ciorbaru, R. & Lederer, E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem. Biophys. Res. Commun. 59, 1317–1325 (1974).
Specter, S., Cimprich, R., Friedman, H. & Chedid, L. Stimulation of an enhanced in vitro immune response by a synthetic adjuvant, muramyl dipeptide. J. Immunol. 120, 487–491 (1978).
Sugimoto, M., Germain, R.N., Chedid, L. & Benacerraf, B. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant, N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP). J. Immunol. 120, 980–982 (1978).
Kleinerman, E.S. et al. Unique histological changes in lung metastases of osteosarcoma patients following therapy with liposomal muramyl tripeptide (CGP 19835A lipid). Cancer Immunol. Immunother. 34, 211–220 (1992).
Ridley, D.S. Histological classification and the immunological spectrum of leprosy. Bull. World Health Organ. 51, 451–465 (1974).
Liu, P.T. et al. Toll-like receptor triggering of a vitamin D–mediated human antimicrobial response. Science 311, 1770–1773 (2006).
Monney, L. et al. TH1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002).
Acknowledgements
We thank P. Liu, B. Bloom and F. Martinon for helpful scientific discussions, D. Vu for technical assistance and A. De Leon for help with the immunolabeling. This work was supported in parts by grants from the US National Institutes of Health (R01s AI022553, AR040312 and AI047868) and the Swiss National Science Foundation (SSMBS, PASMP3-123256).
Author information
Authors and Affiliations
Contributions
R.L.M. and M.S. designed the experiments, interpreted the data and did the majority of the writing. M.S., S.R.K. and P.A.S. performed the experiments. D.J.L. helped with functional microarray analysis. R.M.B.T. provided the leprosy microarray data. M.T.O. performed the confocal imaging. E.K. and T.G.G. performed the bioinformatics analysis. E.N.S. and T.H.R. provided the clinical samples and helped interpret data. S.K. provided reagents and expertise on IL-32. G.C. helped with the conceptual framework and direction of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–25, Supplementary Tables 1–5 and Supplementary Methods (PDF 8957 kb)
Rights and permissions
About this article
Cite this article
Schenk, M., Krutzik, S., Sieling, P. et al. NOD2 triggers an interleukin-32–dependent human dendritic cell program in leprosy. Nat Med 18, 555–563 (2012). https://doi.org/10.1038/nm.2650
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2650
This article is cited by
-
IL-32 induces epithelial-mesenchymal transition by triggering endoplasmic reticulum stress in A549 cells
BMC Pulmonary Medicine (2020)
-
Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation
Scientific Reports (2020)
-
Interleukin 32 expression in human melanoma
Journal of Translational Medicine (2019)
-
Interleukin-32: its role in asthma and potential as a therapeutic agent
Respiratory Research (2018)
-
Triggering through NOD-2 Differentiates Bone Marrow Precursors to Dendritic Cells with Potent Bactericidal activity
Scientific Reports (2016)