Abstract
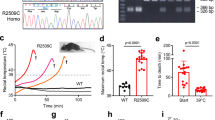
Mice with a knock-in mutation (Y524S) in the type I ryanodine receptor (Ryr1), a mutation analogous to the Y522S mutation that is associated with malignant hyperthermia in humans, die when exposed to short periods of temperature elevation (≥37 °C). We show here that treatment with 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) prevents this heat-induced sudden death in this mouse model. The protection by AICAR is independent of AMP-activated protein kinase (AMPK) activation and results from a newly identified action of the compound on mutant Ryr1 to reduce Ca2+ leak from the sarcoplasmic reticulum to the sarcoplasm. AICAR thus prevents Ca2+-dependent increases in the amount of both reactive oxygen species (ROS) and reactive nitrogen species (RNS) that act to further increase resting Ca2+ concentrations. If unchecked, the temperature-driven increases in resting Ca2+ concentrations and the amounts of ROS and RNS create an amplifying cycle that ultimately triggers sustained muscle contractions, rhabdomyolysis and death. Although antioxidants are effective in reducing this cycle in vitro, only AICAR prevents heat-induced death in vivo. Our findings suggest that AICAR is probably effective in prophylactic treatment of humans with enhanced susceptibility to exercise- and/or heat-induced sudden death associated with RYR1 mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bouchama, A. & Knochel, J.P. Heat stroke. N. Engl. J. Med. 346, 1978–1988 (2002).
Hopkins, P.M., Ellis, F.R. & Halsall, P.J. Evidence for related myopathies in exertional heat stroke and malignant hyperthermia. Lancet 338, 1491–1492 (1991).
Jurkat-Rott, K., McCarthy, T. & Lehmann-Horn, F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve 23, 4–17 (2000).
Wappler, F. et al. Evidence for susceptibility to malignant hyperthermia in patients with exercise-induced rhabdomyolysis. Anesthesiology 94, 95–100 (2001).
Davis, M. et al. Malignant hyperthermia associated with exercise-induced rhabdomyolysis or congenital abnormalities and a novel RYR1 mutation in New Zealand and Australian pedigrees. Br. J. Anaesth. 88, 508–515 (2002).
Treves, S. et al. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscul. Disord. 15, 577–587 (2005).
Rosenberg, H., Davis, M., James, D., Pollock, N. & Stowell, K. Malignant hyperthermia. Orphanet J. Rare Dis. 2, 21 (2007).
Lanner, J.T., Georgiou, D.K., Joshi, A.D. & Hamilton, S.L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Pespect. Bio. 2, a003996 (2010).
Larach, M.G., Gronert, G.A., Allen, G.C.M.D., Brandom, B.W.M.D. & Lehman, E.B.M.S. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth. Analg. 110, 498–507 (2010).
Capacchione, J.F. & Muldoon, S.M. The relationship between exertional heat illness, exertional rhabdomyolysis, and malignant hyperthermia. Anesth. Analg. 109, 1065–1069 (2009).
Groom, L. et al. Identical de novo mutation in the type I ryanodine receptor gene associated with fatal, stress-induced malignant hyperthermia in two unrelated families. Anesthesiology 115, 938–945 (2011).
Vladutiu, G.D. et al. Genetic risk for malignant hyperthermia in non-anesthesia–induced myopathies. Mol. Genet. Metab. 104, 167–173 (2011).
Mackrill, J.J. Ryanodine receptor calcium channels and their partners as drug targets. Biochem. Pharmacol. 79, 1535–1543 (2010).
Grynkiewicz, G., Poenie, M. & Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 (1985).
Konishi, M., Olson, A., Hollingworth, S. & Baylor, S.M. Myoplasmic binding of Fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys. J. 54, 1089–1104 (1988).
Chelu, M.G. et al. Heat- and anesthesia-induced malignant hyperthermia in an RyR1 knock-in mouse. FASEB J. 20, 329–330 (2006).
Durham, W.J. et al. RyR1 S-Nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 133, 53–65 (2008).
Quane, K.A. et al. Mutation screening of the RYR1 gene in malignant hypertherima: detection of a novel Tyr to Ser mutation in a pedigree with associated central core. Genomics 23, 236–239 (1994).
Boncompagni, S . et al. Characterization and temporal development of cores in a mouse model of malignant hyperthermi. Proc. Natl. Acad. Sci. USA 106, 21996–22001 (2009).
Corton, J.M., Gillespie, J.G., Hawley, S.A. & Hardie, D.G. 5-aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 229, 558–565 (1995).
Narkar, V.A. et al. AMPK and PPARΔ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Holmes, B.F., Kurth-Kraczek, E.J. & Winder, W.W. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 87, 1990–1995 (1999).
Pold, R. et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54, 928–934 (2005).
Winder, W.W. et al. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 88, 2219–2226 (2000).
Jørgensen, S.B. et al. Role of AMPK-α2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am. J. Physiol. Endocrinol. Metab. 292, E331–E339 (2007).
Wasserman, K., Beaver, W.L. & Whipp, B.J. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 81 (supp II), 14–30 (1990).
Solberg, G., Robstad, B., Skjønsberg, O.H. & Borchseniu, F. Respiratory gas exchange indices for estimating the anaerobic threshold. J. Sports Sci. Med. 4, 29–36 (2005).
McBride, A., Ghilagaber, S., Nikolaev, A. & Hardie, D.G. The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab. 9, 23–34 (2009).
Fujii, N. et al. AMP-activated protein kinase α2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J. Biol. Chem. 280, 39033–39041 (2005).
Tadaishi, M. et al. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1α mRNA: a role of β2-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 300, E341–E349 (2011).
Fujii, N. et al. Ablation of AMP-activated protein kinase α2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes 57, 2958–2966 (2008).
Barré, L. et al. Genetic model for the chronic activation of skeletal muscle AMP-activated protein kinase leads to glycogen accumulation. Am. J. Physiol. Endocrinol. Metab. 292, E802–E811 (2007).
Meissner, G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J. Biol. Chem. 259, 2365–2374 (1984).
Meissner, G., Rios, E., Tripathy, A. & Pasek, D.A. Kinetics of rapid calcium release by sarcoplasmic reticulum. Effects of calcium, magnesium, and adenine nucleotides. Biochemistry 25, 236–244 (1986).
Meissner, G., Rios, E., Tripathy, A. & Pasek, D.A. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J. Biol. Chem. 272, 1628–1638 (1997).
Terracciano, C., Nogalska, A., Engel, W.K., Wojcik, S. & Askanas, V. In inclusion-body myositis muscle fibers Parkinson-associated DJ-1 is increased and oxidized. Free Radic. Biol. Med. 45, 773–779 (2008).
Korolainen, M.A. et al. Oxidative modification of proteins in the frontal cortex of Alzheimer's disease brain. Neurobiol. Aging 27, 42–53 (2006).
Stamler, J.S. & Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 81, 209–237 (2001).
Wei, L. et al. Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J. 25, 3068–3078 (2011).
Rey, F.E., Cifuentes, M.E., Kiarash, A., Quinn, M.T. & Pagano, P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 89, 408–414 (2001).
Tobin, J.R Challa, V.R., Nelson, T.E. & Sambuughin, N. Malignant hyperthermia and apparent heat stroke. J. Am. Med. Assoc. 286, 168–169 (2001).
Nishio, H. et al. Identification of malignant hyperthermia-susceptible ryanodine receptor type 1 gene (RYR1) mutations in a child who died in a car after exposure to a high environmental temperature. Leg. Med. (Tokyo) 11, 142–143 (2009).
Rock, E. & Kozak-Reiss, G. Effect of halothane on the Ca2+-transport system of surface membranes isolated from normal and malignant hyperthermia pig skeletal muscle. Arch. Biochem. Biophys. 256, 703–707 (1987).
Duke, A.M., Hopkins, P.M., Calaghan, S.C., Halsall, J.P. & Steele, D.S. Store-operated Ca2+ entry in malignant hyperthermia-susceptible human skeletal muscle. J. Biol. Chem. 285, 25645–25653 (2010).
Williams, J.H., Holland, M., Lee, J.C., Ward, C.W. & McGrath, C.J. BAY K 8644 and nifedipine alter halothane but not caffeine contractures of malignant hyperthermic muscle fibers. Am. J. Physiol. 261, R782–R786 (1991).
Pirone, A. et al. Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavα1S-subunit. Am. J. Physiol. Cell Physiol. 299, C1345–C1354 (2010).
Aracena-Parks, P. et al. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J. Biol. Chem. 281, 40354–40368 (2006).
Firuzi, O., Miri, R., Tavakkoli, M. & Saso, L. Antioxidant therapy: current status and future prospects. Curr. Med. Chem. 18, 3871–3888 (2011).
Winder, W.W. & Hardie, D.G. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am. J. Physiol. 270, E299–E304 (1996).
Aracena, P., Tang, W., Hamilton, S.L. & Hidalgo, C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid. Redox Signal. 7, 870–881 (2005).
Lee, H.B., Xu, L. & Meissner, G. Reconstitution of the skeletal muscle ryanodine receptor-Ca2+ release channel protein complex into proteoliposomes. J. Biol. Chem. 269, 13305–13312 (1994).
Liu, Y., Kranias, E.G. & Schneider, M.F. Regulation of Ca2+ handling by phosphorylation status in mouse fast- and slow-twitch skeletal muscle fibers. Am. J. Physiol. 273, C1915–C1924 (1997).
Uto, A., Arai, H. & Ogawa, Y. Reassessment of Fura-2 and the ratio method for determination of intracellular Ca2+ concentrations. Cell Calcium 12, 29–37 (1991).
Acknowledgements
This work was supported by grants from US National Institutes of Health (AR053349), the Department of Defense (DAMD W81XWH-10-2-0117) and the Muscular Dystrophy Association of America. J.T.L. was supported by a postdoc fellowship from The Swedish Research Council. A.D.-A. was supported by a postdoctoral fellowship from The Mexican National Council of Science and Technology (150489). The model shown in Figure 6 was created by S.A. Weldon.
Author information
Authors and Affiliations
Contributions
J.T.L. designed, performed and analyzed the experiments shown in Figures 1a–d and 3a–c, analyzed data, produced Supplementary Figure 1a–f, wrote the initial draft of the paper and edited the final draft. D.K.G. developed the new AMPK assay, designed, performed and analyzed the experiments in Figure 2, wrote an intermediate draft of the paper, prepared the supplementary information, and helped write and edit the final draft of the manuscript. A.D.-A. designed, performed and analyzed the experiments in Figures 4e,f and 5a,b and participated in the writing of the manuscript. A.A. designed, performed and analyzed data from the experiments in Figure 4d. Q.C. made the initial AICAR discovery and performed the experiments in Figure 1e,f and Supplementary Figure 2. A.D.J. generated and analyzed the data in Figure 4g–i and Supplementary Figure 6. Z.C. performed the bilayer experiments. V.Y. designed, performed and analyzed data from the experiments in Supplementary Table 1 and Supplementary Figure 3b,c. J.M.O. performed the experiments shown in Figure 5c,d. C.S.L. designed, performed and analyzed data (using western blots and quantitative RT-PCR) to show that calcium-handling proteins are not changed by the Y524S mutation or by the presence of AICAR. T.O.M. designed the experiments and performed and analyzed many of the pAMPK and AMPK western blots shown in Supplementary Figure 1. A.S. performed all of the mouse dissections, tested the endurance of mice on running wheels, performed indirect calorimetry and contributed to the preparation of the manuscript. K.D. handled all mouse matings and genotyping, performed indirect calorimetry on mice and helped in the manuscript preparation. L.G. provided mice, advised on crucial metabolic experiments and AMPK assays and participated in the writing and critique of the manuscript. I.I.I. designed and performed the experiments in Figure 4a,b and 5e–h, helped in the analysis of the bilayer data and contributed to the manuscript preparation and revision. G.G.R. contributed reagents, supervised, designed and analyzed the experiments to assess the role of NOX and contributed to the manuscript preparation and revision. R.T.D. designed, supervised and analyzed all Ca2+ measurements, critiqued and analyzed all studies and contributed to the manuscript preparation and revision. S.L.H. supervised all experiments, reanalyzed all data for accuracy, plotted all figures and wrote the final draft of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figures 1–6 and Supplementary Methods (PDF 727 kb)
Rights and permissions
About this article
Cite this article
Lanner, J., Georgiou, D., Dagnino-Acosta, A. et al. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat Med 18, 244–251 (2012). https://doi.org/10.1038/nm.2598
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2598
This article is cited by
-
Endurance exercise attenuates juvenile irradiation-induced skeletal muscle functional decline and mitochondrial stress
Skeletal Muscle (2022)
-
Classic and exertional heatstroke
Nature Reviews Disease Primers (2022)
-
Untargeted metabolomics profiling of skeletal muscle samples from malignant hyperthermia susceptible patients
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2021)
-
Ryanodine receptor 1-related disorders: an historical perspective and proposal for a unified nomenclature
Skeletal Muscle (2020)
-
Preclinical model systems of ryanodine receptor 1-related myopathies and malignant hyperthermia: a comprehensive scoping review of works published 1990–2019
Orphanet Journal of Rare Diseases (2020)