Abstract
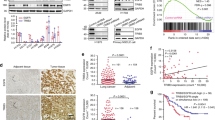
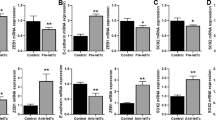
The involvement of the MET oncogene in de novo and acquired resistance of non-small cell lung cancers (NSCLCs) to tyrosine kinase inhibitors (TKIs) has previously been reported, but the precise mechanism by which MET overexpression contributes to TKI-resistant NSCLC remains unclear. MicroRNAs (miRNAs) negatively regulate gene expression, and their dysregulation has been implicated in tumorigenesis. To understand their role in TKI-resistant NSCLCs, we examined changes in miRNA that are mediated by tyrosine kinase receptors. Here we report that miR-30b, miR-30c, miR-221 and miR-222 are modulated by both epidermal growth factor (EGF) and MET receptors, whereas miR-103 and miR-203 are controlled only by MET. We showed that these miRNAs have important roles in gefitinib-induced apoptosis and epithelial-mesenchymal transition of NSCLC cells in vitro and in vivo by inhibiting the expression of the genes encoding BCL2-like 11 (BIM), apoptotic peptidase activating factor 1 (APAF-1), protein kinase C ɛ (PKC-ɛ) and sarcoma viral oncogene homolog (SRC). These findings suggest that modulation of specific miRNAs may provide a therapeutic approach for the treatment of NSCLCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
30 March 2022
Editor’s Note: we are alerting readers that concerns have been raised about the integrity of images in this paper. These issues are currently being reviewed by the editors. A further editorial response will follow upon conclusion of the review.
19 November 2013
In the version of this article initially published, the actin loading control of the western blot in Figure 1a was a rotated duplicate of the actin control in Figure 1h. The authors have not been able to provide the original control data, but they have repeated the experiment and have provided new blots (Fig. 1) that are now published as part of the correction notices linked to the HTML version and attached to the PDF version of the article. The original blots remain in both online versions of the article. In addition, Figure 6g contained a spliced gel that has now been corrected in the HTML and PDF versions of the article.
07 January 2014
A Correction to this paper has been published: https://doi.org/10.1038/nm0114-103
04 October 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41591-022-02044-2
References
Wu, N. et al. MiR-373, a novel regulator of PPP6C, functions as an oncogene in hepatocellular carcinoma. FEBS J. 278, 2044–2054 (2011).
Chan, J.A., Krichevsky, A.M. & Kosik, K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 65, 6029–6033 (2005).
Garofalo, M. et al. MiR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 16, 498–509 (2009).
Cochrane, D.R., Spoleastra Howe, E.N., Nordeen, S.K. & Richer, J.K. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol. Cancer Ther. 8, 1055–1066 (2009).
Molina, J.R., Yang, P., Cassivi, S.D., Schild, S.E. & Adjei, A.A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 83, 584–594 (2008).
Sekido, Y. Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci. 101, 1–6 (2010).
Politi, K., Fan, P.D., Shen, R., Zakowski, M. & Varmus, H. Erlotinib resistance in mouse models of epidermal growth factor receptor–induced lung adenocarcinoma. Dis. Model Mech. 3, 111–119 (2010).
Engelman, J.A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007).
Benedettini, E. et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am. J. Pathol. 177, 415–423 (2010).
Agarwal, S. et al. Association of constitutively activated hepatocyte growth factor receptor (Met) with resistance to a dual EGFR/Her2 inhibitor in non-small-cell lung cancer cells. Br. J. Cancer 100, 941–949 (2009).
Stabile, L.P. et al. Targeting of both the c-Met and EGFR pathways results in additive inhibition of lung tumorigenesis in transgenic mice. Cancers (Basel). 2, 2153–2170 (2010).
Cragg, M.S., Kuroda, J., Puthalakath, H., Huang, D.C. & Strasser, A. Gefitinib-induced killing of NSCLC cell lines expressing mutant EGFR requires BIM and can be enhanced by BH3 mimetics. PLoS Med. 4, 1681–1689 (2007).
Deng, W.G., Knon, J., Ekmekcioglu, S., Poindexter, N.J. & Grimm, E.A. IL-24 gene transfer sensitizes melanoma cells to erlotinib through modulation of the APAF-1 and Akt signaling pathways. Melanoma Res. 21, 44–56 (2010).
Qin, B. et al. Activated Src and Ras induce gefitinib resistance by activation of signaling pathways downstream of epidermal growth factor receptor in human gallbladder adenocarcinoma cells. Cancer Chemother. Pharmacol. 58, 577–584 (2006).
Weisheit, S., Schafer, C., Mertens, C., Berndt, A. & Liebmann, C. PKCɛ acts as negative allosteric modulator of EGF receptor signaling. Cell. Signal. 23, 436–448 (2011).
Turke, A.B. et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 17, 77–88 (2010).
Yoshida, T. et al. Effects of Src inhibitors on cell growth and epidermal growth factor receptor and MET signaling in gefitinib-resistant non-small cell lung cancer cells with acquired MET amplification. Cancer Sci. 101, 167–172 (2010).
Seike, M. et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc. Natl. Acad. Sci. USA 106, 12085–12090 (2009).
Lee, K.H. et al. Epigenetic silencing of microRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology 9, 293–301 (2009).
Viticchiè, G. et al. MiR-203 controls proliferation, migration and invasive potential of prostate cancer cell lines. Cell Cycle 10, 1121–1131 (2011).
Yuan, Y. et al. MicroRNA-203 inhibits cell proliferation by repressing ΔNp63 expression in human esophageal squamous cell carcinoma. BMC Cancer 7, 11–57 (2011).
Sabbah, M. et al. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist. Updat. 11, 123–151 (2008).
Wu, Y. & Zhou, B.P. Snail: more than EMT. Cell Adhes. Migr. 4, 199–203 (2010).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495 (2009).
Chang, C.J. et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 13, 317–323 (2011).
Martello, G. et al. A microRNA targeting dicer for metastasis control. Cell 141, 1195–1207 (2010).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 25, 2380–2388 (2010).
Sos, M.L. et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 15, 3256–3261 (2009).
Meng, F. et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133, 647–658 (2007).
Zhang, J.G. et al. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC). Clin. Chim. Acta 411, 846–852 (2010).
Rho, J.K. et al. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer 63, 219–226 (2009).
Acknowledgements
We thank K. Huebner and S. Lutz for revisions to the paper and P. Fadda and S. Miller for qRT-PCR assistance. We also thank K. Sergott and Ventana Medical Systems for supplying the immunohistochemistry reagents used in this study. M. Nuovo (The Ohio State University Medical Center) kindly provided the photomicroscopy work. We are grateful for research support from The Ohio State University Targeted Investment in Excellence Award, the US National Institutes of Health grant CA113001 and the Kimmel Scholar Award (M.G.).
Author information
Authors and Affiliations
Contributions
M.G. designed the research. M.G., G.R., G.D.L., Y.-J.J., A.N. and F.L. performed the research. G.D.L. and J.S. conducted the mouse experiments. G.N. performed the IHC and ISH experiments. H.A. performed the microarray experiments and analyses. K.P.N. and G.C. provided discussions and advice. J.A.E., M.O. and J.K.R. provided cells with acquired gefitinib resistance. S.V. and L.C. performed bioinformatics analyses. M.G. and C.M.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Methods, Supplementary Figures 1–16 and Supplementary Tables 1 and 2 (PDF 3184 kb)
About this article
Cite this article
Garofalo, M., Romano, G., Di Leva, G. et al. RETRACTED ARTICLE: EGFR and MET receptor tyrosine kinase–altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med 18, 74–82 (2012). https://doi.org/10.1038/nm.2577
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2577
This article is cited by
-
Development of a novel 18F-labeled small molecule probe for PET imaging of mesenchymal epithelial transition receptor expression
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
MicroRNA in lung cancer—a novel potential way for early diagnosis and therapy
Journal of Applied Genetics (2023)
-
MicroRNA-21 guide and passenger strand regulation of adenylosuccinate lyase-mediated purine metabolism promotes transition to an EGFR-TKI-tolerant persister state
Cancer Gene Therapy (2022)
-
Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance
Molecular Cancer (2020)
-
Reduced EGFR and increased miR-221 is associated with increased resistance to temozolomide and radiotherapy in glioblastoma
Scientific Reports (2020)