Abstract
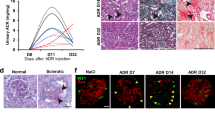
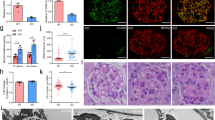
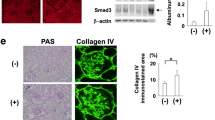
Mechanisms of epithelial cell renewal remain poorly understood in the mammalian kidney, particularly in the glomerulus, a site of cellular damage in chronic kidney disease. Within the glomerulus, podocytes—differentiated epithelial cells crucial for filtration—are thought to lack substantial capacity for regeneration. Here we show that podocytes rapidly lose differentiation markers and enter the cell cycle in adult mice in which the telomerase protein component TERT is conditionally expressed. Transgenic TERT expression in mice induces marked upregulation of Wnt signaling and disrupts glomerular structure, resulting in a collapsing glomerulopathy resembling those in human disease, including HIV-associated nephropathy (HIVAN). Human and mouse HIVAN kidneys show increased expression of TERT and activation of Wnt signaling, indicating that these are general features of collapsing glomerulopathies. Silencing transgenic TERT expression or inhibiting Wnt signaling through systemic expression of the Wnt inhibitor Dkk1 in either TERT transgenic mice or in a mouse model of HIVAN results in marked normalization of podocytes, including rapid cell-cycle exit, re-expression of differentiation markers and improved filtration barrier function. These data reveal an unexpected capacity of podocytes to reversibly enter the cell cycle, suggest that podocyte renewal may contribute to glomerular homeostasis and implicate the telomerase and Wnt–β-catenin pathways in podocyte proliferation and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nadasdy, T., Laszik, Z., Blick, K.E., Johnson, L.D. & Silva, F.G. Proliferative activity of intrinsic cell populations in the normal human kidney. J. Am. Soc. Nephrol. 4, 2032–2039 (1994).
Humphreys, B.D. et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2, 284–291 (2008).
Mundel, P. & Shankland, S.J. Podocyte biology and response to injury. J. Am. Soc. Nephrol. 13, 3005–3015 (2002).
Barisoni, L., Kriz, W., Mundel, P. & D'Agati, V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 10, 51–61 (1999).
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Kriz, W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc. Res. Tech. 57, 189–195 (2002).
Welsh, G.I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12, 329–340 (2010).
Pavenstädt, H. Roles of the podocyte in glomerular function. Am. J. Physiol. Renal Physiol. 278, F173–F179 (2000).
Kriz, W. Progressive renal failure–inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol. Dial. Transplant. 11, 1738–1742 (1996).
Shankland, S.J. & Wolf, G. Cell cycle regulatory proteins in renal disease: role in hypertrophy, proliferation, and apoptosis. Am. J. Physiol. Renal Physiol. 278, F515–F529 (2000).
Barisoni, L. et al. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 58, 137–143 (2000).
Husain, M., D'Agati, V.D., He, J.C., Klotman, M.E. & Klotman, P.E. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS 19, 1975–1980 (2005).
Zuo, Y. et al. HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J. Am. Soc. Nephrol. 17, 2832–2843 (2006).
Rosenstiel, P., Gharavi, A., D'Agati, V. & Klotman, P. Transgenic and infectious animal models of HIV-associated nephropathy. J. Am. Soc. Nephrol. 20, 2296–2304 (2009).
Ding, M. et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat. Med. 12, 1081–1087 (2006).
Korgaonkar, S.N. et al. HIV-1 upregulates VEGF in podocytes. J. Am. Soc. Nephrol. 19, 877–883 (2008).
Ronconi, E. et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 20, 322–332 (2009).
Appel, D. et al. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 20, 333–343 (2009).
Lee, H.W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Allsopp, R.C., Morin, G.B., DePinho, R., Harley, C.B. & Weissman, I.L. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 102, 517–520 (2003).
Batista, L.F. et al. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474, 399–402 (2011).
Sarin, K.Y. et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048–1052 (2005).
Choi, J. et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 4, e10 (2008).
Park, J.I. et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72 (2009).
D'Agati, V. Pathologic classification of focal segmental glomerulosclerosis. Semin. Nephrol. 23, 117–134 (2003).
Imai, E. et al. Glowing podocytes in living mouse: transgenic mouse carrying a podocyte-specific promoter. Exp. Nephrol. 7, 63–66 (1999).
Kaplan, J.M. et al. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat. Genet. 24, 251–256 (2000).
Marshall, C.B. & Shankland, S.J. Cell cycle regulatory proteins in podocyte health and disease. Nephron Exp. Nephrol. 106, e51–e59 (2007).
Nagata, M., Nakayama, K., Terada, Y., Hoshi, S. & Watanabe, T. Cell cycle regulation and differentiation in the human podocyte lineage. Am. J. Pathol. 153, 1511–1520 (1998).
Mundel, P., Reiser, J. & Kriz, W. Induction of differentiation in cultured rat and human podocytes. J. Am. Soc. Nephrol. 8, 697–705 (1997).
Mundel, P. et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 139, 193–204 (1997).
Reiser, J., Kriz, W., Kretzler, M. & Mundel, P. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 11, 1–8 (2000).
Heikkilä, E. et al. Densin and β-catenin form a complex and co-localize in cultured podocyte cell junctions. Mol. Cell. Biochem. 305, 9–18 (2007).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Dickie, P. et al. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 185, 109–119 (1991).
Bruggeman, L.A. et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J. Am. Soc. Nephrol. 11, 2079–2087 (2000).
Bafico, A., Liu, G., Yaniv, A., Gazit, A. & Aaronson, S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3, 683–686 (2001).
Mao, B. et al. LDL-receptor–related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 (2001).
Semënov, M.V. et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11, 951–961 (2001).
Mao, B. et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature 417, 664–667 (2002).
Kuhnert, F. et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA 101, 266–271 (2004).
Iglesias, D.M. et al. Canonical WNT signaling during kidney development. Am. J. Physiol. Renal Physiol. 293, F494–F500 (2007).
Stark, K., Vainio, S., Vassileva, G. & McMahon, A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).
Carroll, T.J., Park, J.S., Hayashi, S., Majumdar, A. & McMahon, A.P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283–292 (2005).
Lancaster, M.A. et al. Impaired Wnt–β-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 15, 1046–1054 (2009).
Lin, S.L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 107, 4194–4199 (2010).
Dai, C. et al. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 20, 1997–2008 (2009).
He, W., Kang, Y.S., Dai, C. & Liu, Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 22, 90–103 (2011).
Kato, H. et al. The Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation and survival. J. Biol. Chem. 286, 26003–26015 (2011).
Sagrinati, C. et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J. Am. Soc. Nephrol. 17, 2443–2456 (2006).
Acknowledgements
We thank P. Chu in the Stanford Comparative Medicine Histology Research Core Laboratory, S. Busque for guidance with nephrectomy, and R. Nusse, Stanford University, for Axin2LacZ/+ mice. M.S. was supported by the Stanford School of Medicine Dean's postdoctoral fellowship and the Stanford Center on Longevity postdoctoral fellowship. This work was supported by US National Institutes of Health grants DK085527 and DK085720 to C.J.K., DK087626 to A.G.G. and CA111691, CA125453, AG033747 and AG036695 to S.E.A.
Author information
Authors and Affiliations
Contributions
M.S., K.Y.S., M.F.P., N.P., F.K., J.L.O., C.J.K., A.G.G., V.D.D.A. and S.E.A. designed the experiments and analyzed data; M.S., K.Y.S., M.F.P., N.P., F.K., W.C., S.A.B., P.C. and E.L. performed the experiments; and M.S. and S.E.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1 and 2 (PDF 16123 kb)
Rights and permissions
About this article
Cite this article
Shkreli, M., Sarin, K., Pech, M. et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med 18, 111–119 (2012). https://doi.org/10.1038/nm.2550
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2550
This article is cited by
-
Novel Pathogenic Mutation of P209L in TRPC6 Gene Causes Adult Focal Segmental Glomerulosclerosis
Biochemical Genetics (2024)
-
Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease
Nature Communications (2022)
-
Telomerase is required for glomerular renewal in kidneys of adult mice
npj Regenerative Medicine (2022)
-
WNT–β-catenin signalling — a versatile player in kidney injury and repair
Nature Reviews Nephrology (2021)
-
Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function
Nature Communications (2019)