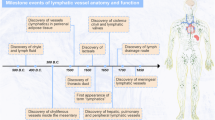
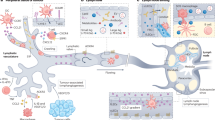
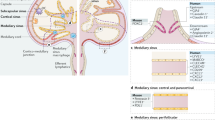
Abstract
Blood vessels form a closed circulatory system, whereas lymphatic vessels form a one-way conduit for tissue fluid and leukocytes. In most vertebrates, the main function of lymphatic vessels is to collect excess protein-rich fluid that has extravasated from blood vessels and transport it back into the blood circulation. Lymphatic vessels have an important immune surveillance function, as they import various antigens and activated antigen-presenting cells into the lymph nodes and export immune effector cells and humoral response factors into the blood circulation. Defects in lymphatic function can lead to lymph accumulation in tissues, dampened immune responses, connective tissue and fat accumulation, and tissue swelling known as lymphedema. This review highlights the most recent developments in lymphatic biology and how the lymphatic system contributes to the pathogenesis of various diseases involving immune and inflammatory responses and its role in disseminating tumor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schulte-Merker, S., Sabine, A. & Petrova, T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 (2011).
Pflicke, H. & Sixt, M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 206, 2925–2935 (2009).
Baluk, P. et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 115, 247–257 (2005).
Dejana, E., Tournier-Lasserve, E. & Weinstein, B.M. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell 16, 209–221 (2009).
Pfeiffer, F. et al. Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur. J. Immunol. 38, 2142–2155 (2008).
Tal, O., et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 208, 2141–2153 (2011).
Norrmén, C., Tammela, T., Petrova, T.V. & Alitalo, K. Biological basis of therapeutic lymphangiogenesis. Circulation 123, 1335–1351 (2011).
Karkkainen, M.J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Wigle, J.T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Albrecht, I. & Christofori, G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int. J. Dev. Biol. 55, 483–494 (2011).
François, M. et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647 (2008).
Srinivasan, R.S. et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 24, 696–707 (2010).
Xu, Y. et al. Neuropilin-2 mediates VEGF-C–induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 188, 115–130 (2010).
Mäkinen, T. et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 19, 397–410 (2005).
Niessen, K. et al. The Notch1-Dll4 signaling pathway regulates mouse postnatal lymphatic development. Blood 118, 1989–1997 (2011).
Zheng, W. et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 118, 1154–1162 (2011).
Augustin, H.G., Koh, G.Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 (2009).
Hogan, B.M. et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat. Genet. 41, 396–398 (2009).
Bos, F.L. et al. CCBE1 Is Essential for Mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ. Res. 109, 486–491 (2011).
Galvagni, F. et al. Endothelial cell adhesion to the extracellular matrix induces c-Src–dependent VEGFR-3 phosphorylation without the activation of the receptor intrinsic kinase activity. Circ. Res. 106, 1839–1848 (2010).
Tammela, T., et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat. Cell Biol. 13, 1202–1213 (2011).
Uhrin, P. et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 115, 3997–4005 (2010).
Bertozzi, C.C. et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116, 661–670 (2010).
Proulx, S.T. et al. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 70, 7053–7062 (2010).
Rasmussen, J.C., Tan, I.C., Marshall, M.V., Fife, C.E. & Sevick-Muraca, E.M. Lymphatic imaging in humans with near-infrared fluorescence. Curr. Opin. Biotechnol. 20, 74–82 (2009).
Vakoc, B.J. et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 15, 1219–1223 (2009).
Song, L., Maslov, K., Shung, K.K. & Wang, L.V. Ultrasound-array–based real-time photoacoustic microscopy of human pulsatile dynamics in vivo. J. Biomed. Opt. 15, 021303 (2010).
Norrmén, C. et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 185, 439–457 (2009).
Kanady, J.D., Dellinger, M.T., Munger, S.J., Witte, M.H. & Simon, A.M. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 354, 253–266 (2011).
Ferrell, R.E. et al. GJC2 missense mutations cause human lymphedema. Am. J. Hum. Genet. 86, 943–948 (2010).
Mellor, R.H. et al. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation 115, 1912–1920 (2007).
Bazigou, E. et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J. Clin. Invest. 121, 2984–2992 (2011).
Karkkainen, M.J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 98, 12677–12682 (2001).
Ostergaard, P., et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 43, 929–931 (2011).
Stanton, A.W., Modi, S., Mellor, R.H., Levick, J.R. & Mortimer, P.S. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat. Res. Biol. 7, 29–45 (2009).
McLaughlin, S.A. et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J. Clin. Oncol. 26, 5213–5219 (2008).
Tammela, T. et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat. Med. 13, 1458–1466 (2007).
Lähteenvuo, M. et al. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation 123, 613–620 (2011).
Cormier, J.N., Rourke, L., Crosby, M., Chang, D. & Armer, J. The surgical treatment of lymphedema: a systematic review of the contemporary literature (2004–2010). Ann. Surg. Oncol. published online, doi:10.1245/s10434-011-2017-4 (24 August 2011).
Saaristo, A.M. et al. Microvascular breast reconstruction and lymph node transfer for postmastectomy lymphedema patients. Ann. Surg. (in the press).
Becker, C., Assouad, J., Riquet, M. & Hidden, G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann. Surg. 243, 313–315 (2006).
Tammela, T. & Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476 (2010).
Rissanen, T.T. et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ. Res. 92, 1098–1106 (2003).
Anisimov, A. et al. Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ. Res. 104, 1302–1312 (2009).
Leppänen, V.M. et al. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc. Natl. Acad. Sci. USA 107, 2425–2430 (2010).
Kisko, K. et al. Structural analysis of vascular endothelial growth factor receptor-2/ligand complexes by small-angle X-ray solution scattering. FASEB J. 25, 2980–2986 (2011).
Leppänen, V.M. et al. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood 117, 1507–1515 (2011).
Vondenhoff, M.F. et al. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J. Immunol. 182, 5439–5445 (2009).
Vondenhoff, M.F. et al. Lymph sacs are not required for the initiation of lymph node formation. Development 136, 29–34 (2009).
Förster, R., Davalos-Misslitz, A.C. & Rot, A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 8, 362–371 (2008).
Wick, N. et al. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am. J. Pathol. 173, 1202–1209 (2008).
Pham, T.H. et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17–27 (2010).
Karikoski, M. et al. Clever-1/Stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation. Eur. J. Immunol. 39, 3477–3487 (2009).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009).
Alvarez, D., Vollmann, E.H. & von Andrian, U.H. Mechanisms and consequences of dendritic cell migration. Immunity 29, 325–342 (2008).
Phan, T.G., Green, J.A., Gray, E.E., Xu, Y. & Cyster, J.G. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10, 786–793 (2009).
Kunder, C.A. et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 206, 2455–2467 (2009).
Cohen, J.N. et al. Lymph node–resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 207, 681–688 (2010).
Kang, S. et al. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood 113, 2605–2613 (2009).
Kataru, R.P. et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance and inflammation resolution. Blood 113, 5650–5659 (2009).
Huggenberger, R. et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117, 4667–4678 (2011).
Angeli, V. et al. B cell–driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 (2006).
Kataru, R.P. et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity 34, 96–107 (2011).
Cueni, L.N. & Detmar, M. The lymphatic system in health and disease. Lymphat. Res. Biol. 6, 109–122 (2008).
von der Weid, P.Y., Rehal, S. & Ferraz, J.G. Role of the lymphatic system in the pathogenesis of Crohn's disease. Curr. Opin. Gastroenterol. 27, 335–341 (2011).
Kerjaschki, D. et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 12, 230–234 (2006).
Nykänen, A.I. et al. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation 121, 1413–1422 (2010).
Albuquerque, R.J. et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 15, 1023–1030 (2009).
Yin, N. et al. Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation 92, 25–30 (2011).
Lämmermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Miteva, D.O. et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 106, 920–931 (2010).
Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 (2010).
Johnson, L.A. & Jackson, D.G. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int. Immunol. 22, 839–849 (2010).
Bao, X. et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33, 817–829 (2010).
Podgrabinska, S. et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1–dependent mechanism. J. Immunol. 183, 1767–1779 (2009).
Vetrano, S. et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut 59, 197–206 (2010).
Gräbner, R. et al. Lymphotoxin Β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged Apoe−/− mice. J. Exp. Med. 206, 233–248 (2009).
van de Pavert, S.A. & Mebius, R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
Muniz, L.R., Pacer, M.E., Lira, S.A. & Furtado, G.C. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J. Immunol. 187, 828–834 (2011).
Mounzer, R.H. et al. Lymphotoxin-Α contributes to lymphangiogenesis. Blood 116, 2173–2182 (2010).
Harvey, N.L. The link between lymphatic function and adipose biology. Ann. NY Acad. Sci. 1131, 82–88 (2008).
Dixon, J.B. Lymphatic lipid transport: sewer or subway? Trends Endocrinol. Metab. 21, 480–487 (2010).
Harvey, N.L. et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072–1081 (2005).
Rutkowski, J.M. et al. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am. J. Pathol. 176, 1122–1129 (2010).
Szuba, A. et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 16, 1985–1987 (2002).
Libby, P., Ridker, P.M. & Hansson, G.K. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 54, 2129–2138 (2009).
Kholová, I. et al. Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions. Eur. J. Clin. Invest. 41, 487–497 (2011).
Nakano, T. et al. Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum. Pathol. 36, 330–340 (2005).
Lim, H.Y. et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am. J. Pathol. 175, 1328–1337 (2009).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Wang, H.W. et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36, 687–693 (2004).
Cheng, F. et al. Virus-induced Notch-MT1-MMP axis leads to lymphatic endothelial-to-mesenchymal transition. Cell Host. Microbe (in the press).
Liu, R. et al. KSHV-induced notch components render endothelial and mural cell characteristics and cell survival. Blood 115, 887–895 (2010).
Zhang, X. et al. Kaposi's sarcoma–associated herpesvirus activation of vascular endothelial growth factor receptor 3 alters endothelial function and enhances infection. J. Biol. Chem. 280, 26216–26224 (2005).
Tvorogov, D. et al. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell 18, 630–640 (2010).
Harari, S., Torre, O. & Moss, J. Lymphangioleiomyomatosis: what do we know and what are we looking for? Eur. Respir. Rev. 20, 34–44 (2011).
McCormack, F.X. et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 364, 1595–1606 (2011).
Seyama, K. et al. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat. Res. Biol. 4, 143–152 (2006).
Fukumura, D., Duda, D.G., Munn, L.L. & Jain, R.K. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206–225 (2010).
Mumprecht, V. et al. In vivo imaging of inflammation- and tumor-induced lymph node lymphangiogenesis by immuno-positron emission tomography. Cancer Res. 70, 8842–8851 (2010).
Leijte, J.A., van der Ploeg, I.M., Valdes Olmos, R.A., Nieweg, O.E. & Horenblas, S. Visualization of tumor blockage and rerouting of lymphatic drainage in penile cancer patients by use of SPECT/CT. J. Nucl. Med. 50, 364–367 (2009).
Giuliano, A.E., et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. J. Am. Med. Assoc. 306, 385–393 (2011).
Louis-Sylvestre, C. et al. Axillary treatment in conservative management of operable breast cancer: dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. J. Clin. Oncol. 22, 97–101 (2004).
Chaffer, C.L. & Weinberg, R.A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Sleeman, J.P., Nazarenko, I. & Thiele, W. Do all roads lead to Rome? Routes to metastasis development. Int. J. Cancer 128, 2511–2526 (2011).
Stoecklein, N.H. & Klein, C.A. Genetic disparity between primary tumours, disseminated tumour cells and manifest metastasis. Int. J. Cancer 126, 589–598 (2010).
Campbell, P.J. et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467, 1109–1113 (2010).
Shields, J.D., Kourtis, I.C., Tomei, A.A., Roberts, J.M. & Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752 (2010).
Kim, M. et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 70, 10411–10421 (2010).
Madsen, C.D. & Sahai, E. Cancer dissemination—lessons from leukocytes. Dev. Cell 19, 13–26 (2010).
Contassot, E., Preynat-Seauve, O., French, L. & Huard, B. Lymph node tumor metastases: more susceptible than primary tumors to CD8+ T cell immune destruction. Trends Immunol. 30, 569–573 (2009).
Kerjaschki, D. et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Invest. 121, 2000–2012 (2011).
Tammela, T. et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 (2008).
Roberts, N. et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 66, 2650–2657 (2006).
Caunt, M. et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 13, 331–342 (2008).
Hooper, A.T. et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274 (2009).
Zbytek, B. et al. Current concepts of metastasis in melanoma. Expert Rev. Dermatol. 3, 569–585 (2008).
Tammela, T. et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci. Transl. Med. 3, 69ra11 (2011).
Goyal, S., Chauhan, S.K. & Dana, R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch. Ophthalmol. published online, doi:10.1001/archophthalmol.2011.266 (12 September 2011).
Koch, S., Tugues, S., Li, X., Gualandi, L. & Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 (2011).
Saharinen, P. et al. Claudin-like protein 24 interacts with the VEGFR-2 and VEGFR-3 pathways and regulates lymphatic vessel development. Genes Dev. 24, 875–880 (2010).
Yang, Y., Xie, P., Opatowsky, Y. & Schlessinger, J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc. Natl. Acad. Sci. USA 107, 1906–1911 (2010).
Kendrew, J. et al. An antibody targeted to VEGFR-2 Ig domains 4–7 inhibits VEGFR-2 activation and VEGFR-2–dependent angiogenesis without affecting ligand binding. Mol. Cancer Ther. 10, 770–783 (2011).
Koh, Y.J. et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis and vascular leakage. Cancer Cell 18, 171–184 (2010).
Hashizume, H. et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 70, 2213–2223 (2010).
Brown, J.L. et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol. Cancer Ther. 9, 145–156 (2010).
Acknowledgements
I am grateful to M. Bry, L. Eklund, S. Jalkanen, D. Kerjaschki, G.Y. Koh, V.-M. Leppänen, T. Mäkinen, A. Nykänen, M. Öhman, P. Ojala, P. Saharinen, M. Swartz and T. Tammela for useful discussions of the topics of this review. The lymph node images in Figure 5a were kindly provided by D. Kerjaschki and by G.Y. Koh, and draft figures were produced by H. Schmidt. The studies in my laboratory are currently supported by the Academy of Finland, the Finnish Cancer Organisations, the Association for International Cancer Research, the Sigrid Juselius Foundation, Seventh Framework Program of the European Union (ERC Advanced Grant), Finnish Foundation for Cardiovascular Research and Biocentrum Finland. I apologize to the many authors whose important work could not be cited because of space restrictions and the focus on the recent developments; older references appear in the many excellent reviews that have been cited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K.A. is chairman of the Scientific Advisory Board of Circadian Technologies Limited. K.A. is also a consultant for Laurantis Pharma Oy.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 268 kb)
Rights and permissions
About this article
Cite this article
Alitalo, K. The lymphatic vasculature in disease. Nat Med 17, 1371–1380 (2011). https://doi.org/10.1038/nm.2545
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2545
This article is cited by
-
Copper nanoparticles and silver nanoparticles impair lymphangiogenesis in zebrafish
Cell Communication and Signaling (2024)
-
The role of lymphatic vessels in corneal fluid homeostasis and wound healing
Journal of Ophthalmic Inflammation and Infection (2024)
-
The development of early human lymphatic vessels as characterized by lymphatic endothelial markers
The EMBO Journal (2024)
-
Anti-lymphangiogenesis for boosting drug accumulation in tumors
Signal Transduction and Targeted Therapy (2024)
-
Current and Developing Lymphatic Imaging Approaches for Elucidation of Functional Mechanisms and Disease Progression
Molecular Imaging and Biology (2024)