Abstract
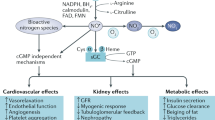
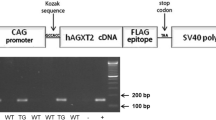
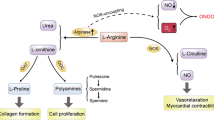
Nitric oxide (NO) is crucial in diverse physiological and pathological processes. We show that a hypomorphic mouse model of argininosuccinate lyase (encoded by Asl) deficiency has a distinct phenotype of multiorgan dysfunction and NO deficiency. Loss of Asl in both humans and mice leads to reduced NO synthesis, owing to both decreased endogenous arginine synthesis and an impaired ability to use extracellular arginine for NO production. Administration of nitrite, which can be converted into NO in vivo, rescued the manifestations of NO deficiency in hypomorphic Asl mice, and a nitric oxide synthase (NOS)-independent NO donor restored NO-dependent vascular reactivity in humans with ASL deficiency. Mechanistic studies showed that ASL has a structural function in addition to its catalytic activity, by which it contributes to the formation of a multiprotein complex required for NO production. Our data demonstrate a previously unappreciated role for ASL in NOS function and NO homeostasis. Hence, ASL may serve as a target for manipulating NO production in experimental models, as well as for the treatment of NO-related diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Flam, B.R., Hartmann, P.J., Harrell-Booth, M., Solomonson, L.P. & Eichler, D.C. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide 5, 187–197 (2001).
Mori, M. & Gotoh, T. Arginine metabolic enzymes, nitric oxide and infection. J. Nutr. 134, 2820S–2825S (2004).
Morris, S.M. Jr. Arginine metabolism: boundaries of our knowledge. J. Nutr. 137, 1602S–1609S (2007).
Krivitzky, L. et al. Intellectual, adaptive and behavioral functioning in children with urea cycle disorders. Pediatr. Res. 66, 96–101 (2009).
Scaglia, F. et al. Clinical consequences of urea cycle enzyme deficiencies and potential links to arginine and nitric oxide metabolism. J. Nutr. 134, 2775S–2782S (2004).
Zimmermann, A., Bachmann, C. & Baumgartner, R. Severe liver fibrosis in argininosuccinic aciduria. Arch. Pathol. Lab. Med. 110, 136–140 (1986).
Mori, T. et al. Progressive liver fibrosis in late-onset argininosuccinate lyase deficiency. Pediatr. Dev. Pathol. 5, 597–601 (2002).
Erez, A., Nagamani, S.C. & Lee, B. Argininosuccinate lyase deficiency-argininosuccinic aciduria and beyond. Am. J. Med. Genet. C. Semin. Med. Genet. 157, 45–53 (2011).
Brunetti-Pierri, N., Erez, A., Shchelochkov, O., Craigen, W. & Lee, B. Systemic hypertension in two patients with ASL deficiency: A result of nitric oxide deficiency? Mol. Genet. Metab. 98, 195–197 (2009).
Fakler, C.R., Kaftan, H.A. & Nelin, L.D. Two cases suggesting a role for the L-arginine nitric oxide pathway in neonatal blood pressure regulation. Acta Paediatr. 84, 460–462 (1995).
Pearson, D.L. et al. Neonatal pulmonary hypertension–urea-cycle intermediates, nitric oxide production and carbamoyl-phosphate synthetase function. N. Engl. J. Med. 344, 1832–1838 (2001).
Summar, M.L. et al. Relationship between carbamoyl-phosphate synthetase genotype and systemic vascular function. Hypertension 43, 186–191 (2004).
Nagasaka, H. et al. Evaluation of endogenous nitric oxide synthesis in congenital urea cycle enzyme defects. Metabolism 58, 278–282 (2009).
Reid Sutton, V.P.Y., Davis, E.C. & Craigen, W.J. A mouse model of argininosuccinic aciduria: biochemical characterization. Mol. Genet. Metab. 78, 11–16 (2003).
de Jonge, W.J. et al. Overexpression of arginase I in enterocytes of transgenic mice elicits a selective arginine deficiency and affects skin, muscle and lymphoid development. Am. J. Clin. Nutr. 76, 128–140 (2002).
Auron, A. & Brophy, P.D. Hyperammonemia in review: pathophysiology, diagnosis and treatment. Pediatr. Nephrol. published online, doi:10.1007/s00467-011-1838-5 (23 March 2011).
Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Aspects Med. 26, 33–65 (2005).
Tojo, A.O.M. & Fujita, T. Role of macula densa neuronal nitric oxide synthase in renal diseases. Med. Mol. Morphol. 39, 2–7 (2006).
Zweier, J.L., Wang, P., Samouilov, A. & Kuppusamy, P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1, 804–809 (1995).
Cosby, K. et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505 (2003).
Thomas, G., Hecker, M. & Ramwell, P.W. Vascular activity of polycations and basic amino acids: L-arginine does not specifically elicit endothelium-dependent relaxation. Biochem. Biophys. Res. Commun. 158, 177–180 (1989).
Gold, M.E., Wood, K.S., Byrns, R.E., Buga, G.M. & Ignarro, L.J. L-arginine-dependent vascular smooth muscle relaxation and cGMP formation. Am. J. Physiol. 259, H1813–H1821 (1990).
Bode-Böger, S.M., Scalera, F. & Ignarro, L.J. The l-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 114, 295–306 (2007).
Cals-Grierson, M.M. & Ormerod, A.D. Nitric oxide function in the skin. Nitric Oxide 10, 179–193 (2004).
Shimizu, Y., Sakai, M., Umemura, Y. & Ueda, H. Immunohistochemical localization of nitric oxide synthase in normal human skin: expression of endothelial-type and inducible-type nitric oxide synthase in keratinocytes. J. Dermatol. 24, 80–87 (1997).
Wang, R., Ghahary, A., Shen, Y.J., Scott, P.G. & Tredget, E.E. Human dermal fibroblasts produce nitric oxide and express both constitutive and inducible nitric oxide synthase isoforms. J. Invest. Dermatol. 106, 419–427 (1996).
Kurotobi, S. et al. Impaired vascular endothelium–dependent relaxation in Henoch-Schonlein purpura. Pediatr. Nephrol. 19, 138–143 (2004).
Flam, B.R., Hartmann, P.J., Harrell-Booth, M., Solomonson, L.P. & Eichler, D.C. Caveolar localization of arginine regeneration enzymes, argininosuccinate synthase, and lyase, with endothelial nitric oxide synthase. Nitric Oxide 5, 187–197 (2001).
Solomonson, L.P., Flam, B.R., Pendleton, L.C., Goodwin, B.L. & Eichler, D.C. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J. Exp. Biol. 206, 2083–2087 (2003).
Oyadomari, S. et al. Coinduction of endothelial nitric oxide synthase and arginine recycling enzymes in aorta of diabetic rats. Nitric Oxide 5, 252–260 (2001).
Li, C., Huang, W., Harris, M.B., Goolsby, J.M. & Venema, R.C. Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem. J. 386, 567–574 (2005).
Trevisson, E. et al. Functional complementation in yeast allows molecular characterization of missense argininosuccinate lyase mutations. J. Biol. Chem. 284, 28926–28934 (2009).
Trevisson, E. et al. Argininosuccinate lyase deficiency: mutational spectrum in Italian patients and identification of a novel ASL pseudogene. Hum. Mutat. 28, 694–702 (2007).
Trevisson, E. et al. Functional complementation in yeast allows molecular characterization of missense argininosuccinate lyase mutations. J. Biol. Chem. 284, 28926–28934 (2009).
Trevisson, E. et al. Argininosuccinate lyase deficiency: mutational spectrum in Italian patients and identification of a novel ASL pseudogene. Hum. Mutat. 28, 694–702 (2007).
Vallee, F., Turner, M.A., Lindley, P.L. & Howell, P.L. Crystal structure of an inactive duck delta II crystallin mutant with bound argininosuccinate. Biochemistry 38, 2425–2434 (1999).
Lee, H.J., Chiou, S.H. & Chang, G.G. Biochemical characterization and kinetic analysis of duck delta-crystallin with endogenous argininosuccinate lyase activity. Biochem. J. 283, 597–603 (1992).
Liu, P., Jenkins, N.A. & Copeland, N.G. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476–484 (2003).
Morello, R. et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127, 291–304 (2006).
Skarnes, W.C. et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474, 337–342 (2011).
Bryan, N.S. et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc. Natl. Acad. Sci. USA 101, 4308–4313 (2004).
Wang, X. et al. Measurement of nitric oxide levels in the red cell: validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J. Biol. Chem. 281, 26994–27002 (2006).
Heller, R. et al. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J. Biol. Chem. 276, 40–47 (2001).
Dal Pra, I., Chiarini, A., Nemeth, E.F., Armato, U. & Whitfield, J.F. Roles of Ca2+ and the Ca2+-sensing receptor (CASR) in the expression of inducible NOS (nitric oxide synthase)-2 and its BH4 (tetrahydrobiopterin)-dependent activation in cytokine-stimulated adult human astrocytes. J. Cell. Biochem. 96, 428–438 (2005).
Marley, R., Feelisch, M., Holt, S. & Moore, K. A chemiluminescense-based assay for S-nitrosoalbumin and other plasma S-nitrosothiols. Free Radic. Res. 32, 1–9 (2000).
Bryan, N.S. & Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 43, 645–657 (2007).
Acknowledgements
This work was supported by the US National Institutes of Health (DK54450, RR19453, RR00188, GM90310 to B.L., GM07526 and DK081735 to A.E., HL75511 to J.L.A., RR024173 to J.C.M. and DK37175 to W.E.M.). We acknowledge and thank the clinical efforts of M. Mullins, S. Carter, A. Tran, J. Stuff, W. Martinek and the Texas Children's Hospital General Clinical Research Center nursing staff. The mutated human ASL–encoding plasmids were kindly provided by L. Salviati from the Department of Pediatrics, University of Padova, Padova, Italy. We thank the families of the subjects for their kind participation. We greatly appreciate the technical contributions of M. Jiang, E. Munivez, B. Dawson, G. Sule and J. Zhang. We thank L. Castillo for helpful discussions. Figures were produced using Servier Medical Art. A.E., S.C.S.N. and O.A.S. are awardees of the National Urea Cycle Disorder Foundation Research Fellowship. O.A.S. is an awardee of the O'Malley Fellowship of the Urea Cycle Disorders Rare Disease Clinical Research Network. N.S.B. is supported by the American Heart Association-National 0735042N. P.M.C. is an awardee of the Canadian Institute of Health Research clinician-scientist training award.
Author information
Authors and Affiliations
Contributions
A.E. generated the mouse models, performed most of the experiments and wrote the manuscript. S.C.S.N. performed the human in vivo and in vitro experiments. O.A.S. conducted the mouse therapy experiments. M.H.P. conducted the immunoprecipitation experiments. P.M.C. helped conducting the mutated ASL experiments. Y.C. generated the mouse models. H.K.G. analyzed the NOx data and helped with the ELISA experiments. L.L. performed western blot experiments. A.M. conducted the human studies. T.K.B. conducted the RT-PCR experiments. J.O.B. performed the histological analysis. H.Z. performed western blot and immunoprecipitation experiments. Y.T. conducted the vascular ring experiments. A.K.R. helped with the blood pressure analysis. M.S. contributed to the conception of the hypothesis. W.E.O. analyzed the biochemical data. D.G.H. contributed to our understanding regarding NOS function. W.E.M. conducted the vessel reactivity assay and helped with assessing creatinine clearance. J.C.M. performed the labeled isotope studies. J.L.A. supervised the experiments performed on the Asl hypomorphic lungs, helped in key analyses and helped in revising of the manuscript. N.S.B. helped with the NOx and NOS data analysis and supervised related experiments. B.L. led and supervised the project through all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 3449 kb)
Rights and permissions
About this article
Cite this article
Erez, A., Nagamani, S., Shchelochkov, O. et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med 17, 1619–1626 (2011). https://doi.org/10.1038/nm.2544
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2544
This article is cited by
-
ASS1 and ASL suppress growth in clear cell renal cell carcinoma via altered nitrogen metabolism
Cancer & Metabolism (2021)
-
Potential role of microbiome in Chronic Fatigue Syndrome/Myalgic Encephalomyelits (CFS/ME)
Scientific Reports (2021)
-
ASL expression in ALDH1A1+ neurons in the substantia nigra metabolically contributes to neurodegenerative phenotype
Human Genetics (2021)
-
Vascular Metabolic Mechanisms of Pulmonary Hypertension
Current Medical Science (2020)
-
Free Radical Scavengers Prevent Argininosuccinic Acid-Induced Oxidative Stress in the Brain of Developing Rats: a New Adjuvant Therapy for Argininosuccinate Lyase Deficiency?
Molecular Neurobiology (2020)