Abstract
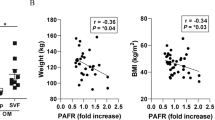
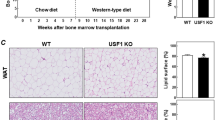
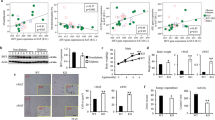
Tissue factor, the initiator of the coagulation cascade, mediates coagulation factor VIIa–dependent activation of protease-activated receptor 2 (PAR2). Here we delineate a role for this signaling pathway in obesity and its complications. Mice lacking PAR2 (F2rl1) or the cytoplasmic domain of tissue factor were protected from weight gain and insulin resistance induced by a high-fat diet. In hematopoietic cells, genetic ablation of tissue factor–PAR2 signaling reduced adipose tissue macrophage inflammation, and specific pharmacological inhibition of macrophage tissue factor signaling rapidly ameliorated insulin resistance. In contrast, nonhematopoietic cell tissue factor–VIIa-PAR2 signaling specifically promoted obesity. Mechanistically, adipocyte tissue factor cytoplasmic domain–dependent VIIa signaling suppressed Akt phosphorylation with concordant adverse transcriptional changes of key regulators of obesity and metabolism. Pharmacological blockade of adipocyte tissue factor in vivo reversed these effects of tissue factor–VIIa signaling and rapidly increased energy expenditure. Thus, inhibition of tissue factor signaling is a potential therapeutic avenue for improving impaired metabolism and insulin resistance in obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kadowaki, T. et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 (2006).
Oh, D.Y. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Ouchi, N. et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454–457 (2010).
Odegaard, J.I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Rajagopal, S., Rajagopal, K. & Lefkowitz, R.J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 (2010).
Wang, P. & DeFea, K.A. Protease-activated receptor-2 simultaneously directs β-arrestin-1–dependent inhibition and Gαq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry 45, 9374–9385 (2006).
Luan, B. et al. Deficiency of a β-arrestin-2 signal complex contributes to insulin resistance. Nature 457, 1146–1149 (2009).
Wang, P., Kumar, P., Wang, C. & DeFea, K.A. Differential regulation of class IA phosphoinositide 3-kinase catalytic subunits p110 α and β by protease-activated receptor 2 and β-arrestins. Biochem. J. 408, 221–230 (2007).
Zoudilova, M. et al. β-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 282, 20634–20646 (2007).
Wang, P., Jiang, Y., Wang, Y., Shyy, J.Y. & DeFea, K.A. β-arrestin inhibits CAMKKβ-dependent AMPK activation downstream of protease-activated-receptor-2. BMC Biochem. 11, 36 (2010).
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
De Taeye, B., Smith, L.H. & Vaughan, D.E. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr. Opin. Pharmacol. 5, 149–154 (2005).
Gerrits, A.J., Koekman, C.A., Haeften, V.A.N.T.W. & Akkerman, J.W. Increased tissue factor expression in diabetes mellitus type 2 monocytes caused by insulin resistance. J. Thromb. Haemost. 9, 873–875 (2011).
Diamant, M. et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 106, 2442–2447 (2002).
Kopp, C.W. et al. Weight loss reduces tissue factor in morbidly obese patients. Obes. Res. 11, 950–956 (2003).
Samad, F., Pandey, M. & Loskutoff, D.J. Tissue factor gene expression in the adipose tissues of obese mice. Proc. Natl. Acad. Sci. USA 95, 7591–7596 (1998).
Dorfleutner, A., Hintermann, E., Tarui, T., Takada, Y. & Ruf, W. Cross-talk of integrin α3β1 and tissue factor in cell migration. Mol. Biol. Cell 15, 4416–4425 (2004).
Schaffner, F. et al. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood 116, 6106–6113 (2010).
Redecha, P., Franzke, C.W., Ruf, W., Mackman, N. & Girardi, G. Neutrophil activation by the tissue factor/factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J. Clin. Invest. 118, 3453–3461 (2008).
Ahamed, J. et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc. Natl. Acad. Sci. USA 103, 13932–13937 (2006).
Versteeg, H.H. et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood 111, 190–199 (2008).
Olefsky, J.M. & Glass, C.K. Macrophages, inflammation and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008).
Cinti, S. et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 (2005).
Guha, M. & Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 13, 85–94 (2001).
Randolph, G.J., Luther, T., Albrecht, S., Magdolen, V. & Muller, W.A. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood 92, 4167–4177 (1998).
Ahamed, J. et al. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood 109, 5251–5259 (2007).
Noorbakhsh, F. et al. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Exp. Med. 203, 425–435 (2006).
Odegaard, J.I. et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 (2008).
Saberi, M. et al. Hematopoietic cell-specific deletion of Toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 (2009).
Liu, L. et al. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 117, 1679–1689 (2007).
Pickersgill, L., Litherland, G.J., Greenberg, A.S., Walker, M. & Yeaman, S.J. Key role for ceramides in mediating insulin resistance in human muscle cells. J. Biol. Chem. 282, 12583–12589 (2007).
Snyder, L.A. et al. Expression of human tissue factor under the control of the mouse tissue factor promoter mediates normal hemostasis in knock-in mice. J. Thromb. Haemost. 6, 306–314 (2008).
Bradshaw, G. et al. Facilitated replacement of Kupffer cells expressing a paraoxonase-1 transgene is essential for ameliorating atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 102, 11029–11034 (2005).
Mackman, N., Sawdey, M.S., Keeton, M.R. & Loskutoff, D.J. Murine tissue factor gene expression in vivo: tissue and cell specificity and regulation by lipopolysaccharide. Am. J. Pathol. 143, 76–84 (1993).
Samad, F., Pandey, M. & Loskutoff, D.J. Regulation of tissue factor gene expression in obesity. Blood 98, 3353–3358 (2001).
Venugopal, J., Hanashiro, K., Yang, Z.Z. & Nagamine, Y. Identification and modulation of a caveolae-dependent signal pathway that regulates plasminogen activator inhibitor-1 in insulin-resistant adipocytes. Proc. Natl. Acad. Sci. USA 101, 17120–17125 (2004).
Kidani, T. et al. Bisphenol A downregulates Akt signaling and inhibits adiponectin production and secretion in 3T3–L1 adipocytes. J. Atheroscler. Thromb. 17, 834–843 (2010).
Matsuzawa, Y. Adiponectin: a key player in obesity related disorders. Curr. Pharm. Des. 16, 1896–1901 (2010).
Qi, Y. et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 10, 524–529 (2004).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8, 1288–1295 (2002).
Jäger, S., Handschin, C., St-Pierre, J. & Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 104, 12017–12022 (2007).
Lee, Y. et al. PPAR α is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc. Natl. Acad. Sci. USA 99, 11848–11853 (2002).
Uysal, K.T., Wiesbrock, S.M., Marino, M.W. & Hotamisligil, G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389, 610–614 (1997).
Guerre-Millo, M. et al. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 275, 16638–16642 (2000).
Surwit, R.S. et al. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc. Natl. Acad. Sci. USA 95, 4061–4065 (1998).
Fleury, C. et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 15, 269–272 (1997).
Hotamisligil, G.S., Shargill, N.S. & Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Hotamisligil, G.S., Budavari, A., Murray, D. & Spiegelman, B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-α. J. Clin. Invest. 94, 1543–1549 (1994).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Lumeng, C.N., Bodzin, J.L. & Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Steinbrecher, K.A. et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin αMβ2 engagement of fibrinogen. Cancer Res. 70, 2634–2643 (2010).
Smiley, S.T., King, J.A. & Hancock, W.W. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J. Immunol. 167, 2887–2894 (2001).
Flick, M.J. et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest. 113, 1596–1606 (2004).
Melis, E. et al. Targeted deletion of the cytosolic domain of tissue factor in mice does not affect development. Biochem. Biophys. Res. Commun. 286, 580–586 (2001).
Damiano, B.P. et al. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J. Pharmacol. Exp. Ther. 288, 671–678 (1999).
Yang, G. et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297, E211–E224 (2009).
Samad, F., Yamamoto, K. & Loskutoff, D.J. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-α and lipopolysaccharide. J. Clin. Invest. 97, 37–46 (1996).
Acknowledgements
This study was supported by US National Institutes of Health grants HL71146, HL104232 (F.S.) and HL77753 (W.R.), a grant from the Diabetes National Research Group (F.S.) and a postdoctoral fellowship from the American Heart Association (C.F.-F.). We thank M. Anderson (Johnson & Johnson Pharmaceutical Research and Development, Radnor, Pennsylvania, USA) for TFKI mice, C. Biazak, J. Royce, N. Vu and R. Zubairi for technical assistance, A.J. Roberts for assistance with metabolic studies and C. Johnson for preparation of figures.
Author information
Authors and Affiliations
Contributions
L.B. carried out metabolic, gene expression and in vitro experiments and analyzed data. C.F.-F. generated bone marrow chimeras, carried out FACS analysis and analyzed data. G.Y. carried out in vivo and in vitro Akt activation assays. W.R. and F.S. designed experiments, analyzed data, wrote the manuscript and share the last authorship.
Corresponding authors
Ethics declarations
Competing interests
W.R. and F.S. have a US patent application on the use of tissue factor–specific antibodies to ameliorate insulin resistance and obesity.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 616 kb)
Rights and permissions
About this article
Cite this article
Badeanlou, L., Furlan-Freguia, C., Yang, G. et al. Tissue factor–protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med 17, 1490–1497 (2011). https://doi.org/10.1038/nm.2461
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2461
This article is cited by
-
Identification of the potential regulatory interactions in rheumatoid arthritis through a comprehensive analysis of lncRNA-related ceRNA networks
BMC Musculoskeletal Disorders (2023)
-
Protease-activated receptor 2 attenuates doxorubicin-induced apoptosis in colon cancer cells
Journal of Cell Communication and Signaling (2023)
-
Immunoreactivity of Plasminogen Activator Inhibitor 1 and Its Correlation with Dysmenorrhea and Lesional Fibrosis in Adenomyosis
Reproductive Sciences (2021)
-
Activation of PAR2 by tissue factor induces the release of the PTEN from MAGI proteins and regulates PTEN and Akt activities
Scientific Reports (2020)
-
Inhibition of Serine Protease Activity Protects Against High Fat Diet-Induced Inflammation and Insulin Resistance
Scientific Reports (2020)