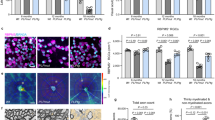
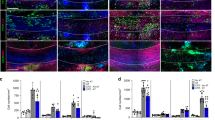
Abstract
In multiple sclerosis, activated CD4+ T cells initiate an immune response in the brain and spinal cord, resulting in demyelination, degeneration and progressive paralysis. Repulsive guidance molecule-a (RGMa) is an axon guidance molecule that has a role in the visual system and in neural tube closure. Our study shows that RGMa is expressed in bone marrow–derived dendritic cells (BMDCs) and that CD4+ T cells express neogenin, a receptor for RGMa. Binding of RGMa to CD4+ T cells led to activation of the small GTPase Rap1 and increased adhesion of T cells to intracellular adhesion molecule-1 (ICAM-1). Neutralizing antibodies to RGMa attenuated clinical symptoms of mouse myelin oligodendrocyte glycoprotein (MOG)-induced experimental autoimmune encephalomyelitis (EAE) and reduced invasion of inflammatory cells into the CNS. Silencing of RGMa in MOG-pulsed BMDCs reduced their capacity to induce EAE following adoptive transfer to naive C57BL/6 mice. CD4+ T cells isolated from mice treated with an RGMa-specific antibody showed diminished proliferative responses and reduced interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4 and IL-17 secretion. Incubation of PBMCs from patients with multiple sclerosis with an RGMa-specific antibody reduced proliferative responses and pro-inflammatory cytokine expression. These results demonstrate that an RGMa-specific antibody suppresses T cell responses, and suggest that RGMa could be a promising molecular target for the treatment of multiple sclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Trapp, B.D., Ransohoff, R.M., Fisher, E. & Rudick, R. Neurodegeneration in multiple sclerosis: relationship to neurological disability. Neuroscientist 5, 48–57 (1999).
Smith, A. et al. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 218, 135–146 (2007).
Katagiri, K. et al. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 20, 1956–1969 (2000).
Reedquist, K.A. et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol. 148, 1151–1158 (2000).
Katagiri, K., Hattori, M., Minato, N. & Kinashi, T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol. Cell. Biol. 22, 1001–1015 (2002).
Stahl, B., Müller, B., von Boxberg, Y., Cox, E.C. & Bonhoeffer, F. Biochemical characterization of a putative axonal guidance molecule of the chick visual system. Neuron 5, 735–743 (1990).
Yamashita, T., Mueller, B.K. & Hata, K. Neogenin and repulsive guidance molecule signaling in the central nervous system. Curr. Opin. Neurobiol. 17, 29–34 (2007).
Suga, K. et al. CD98 induces LFA-1–mediated cell adhesion in lymphoid cells via activation of Rap1. FEBS Lett. 489, 249–253 (2001).
Serafini, B. et al. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake and interaction with proliferating T cells. J. Neuropathol. Exp. Neurol. 65, 124–141 (2006).
Hata, K. et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J. Cell Biol. 173, 47–58 (2006).
McRae, B.L., Vanderlugt, C.L., Dal Canto, M.C. & Miller, S.D. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 182, 75–85 (1995).
McMahon, E.J. et al. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Duffield, J.S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Nohra, R. et al. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 11, 279–293 (2010).
Trapp, B.D., Ransohoff, R. & Rudick, R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 12, 295–302 (1999).
Onuki, M., Ayers, M.M., Bernard, C.C. & Orian, J.M. Axonal degeneration is an early pathological feature in autoimmune-mediated demyelination in mice. Microsc. Res. Tech. 52, 731–739 (2001).
Karnezis, T. et al. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat. Neurosci. 7, 736–744 (2004).
Mi, S. et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13, 1228–1233 (2007).
Polman, C.H. et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 58, 840–846 (2005).
Acknowledgements
This work was supported by a Grant-in-Aid for Young Scientists (S) from the Japan Society for the Promotion of Sciences (19679007) to T.Y., and a Grant-in-Aid from Ministry of Health, Labour and Welfare to T.Y. We thank H. Hayakawa and members of the Yamashita laboratory for fruitful discussion and help.
Author information
Authors and Affiliations
Contributions
T.K. performed preliminary experiments for expression analysis, behavioral and histological analysis of EAE, and cytokine production, and contributed to conceiving the study. Later, R.M. took over the work and performed all experiments, with the exception of the portions indicated below. Y.N. performed EAE induction, adoptive transfer experiments, immunohistochemical analyses and spinal cord injury experiments. Y.F. performed the lymphocyte binding assay, Rap1 activity assay and cytokine analysis. M.M., J.T. and S.K. performed experiments with PBMCs. T.O. helped with irradiation experiments. M.M., T.A., J.T., M.Y., H.M. and S.K. performed experiments with autopsy samples. T.K and T.Y. conceived the project and developed the hypothesis. T.K, R.M., A.K. and T.Y. designed the experiments. A.K. and T.O. discussed the hypothesis and helped with data interpretation. T.Y. coordinated and directed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Methods (PDF 2200 kb)
Rights and permissions
About this article
Cite this article
Muramatsu, R., Kubo, T., Mori, M. et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat Med 17, 488–494 (2011). https://doi.org/10.1038/nm.2321
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2321
This article is cited by
-
Mining the microbiota to identify gut commensals modulating neuroinflammation in a mouse model of multiple sclerosis
Microbiome (2022)
-
Modulation of Microglia M2 Polarization and Alleviation of Hippocampal Neuron Injury By MiR-106b-5p/RGMa in a Mouse Model of Status Epilepticus
Inflammation (2022)
-
Age-dependent decline in remyelination capacity is mediated by apelin–APJ signaling
Nature Aging (2021)
-
miR-106b-5p upregulation is associated with microglial activation and inflammation in the mouse hippocampus following status epilepticus
Experimental Brain Research (2021)
-
Niche derived netrin-1 regulates hematopoietic stem cell dormancy via its receptor neogenin-1
Nature Communications (2021)