Abstract
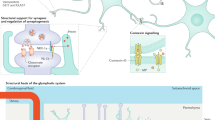
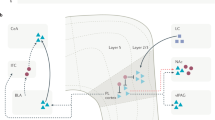
Chronic pain is a major challenge to clinical practice and basic science. The peripheral and central neural networks that mediate nociception show extensive plasticity in pathological disease states. Disease-induced plasticity can occur at both structural and functional levels and is manifest as changes in individual molecules, synapses, cellular function and network activity. Recent work has yielded a better understanding of communication within the neural matrix of physiological pain and has also brought important advances in concepts of injury-induced hyperalgesia and tactile allodynia and how these might contribute to the complex, multidimensional state of chronic pain. This review focuses on the molecular determinants of network plasticity in the central nervous system (CNS) and discusses their relevance to the development of new therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Darwin, C. The Expression of the Emotions in Man and Animals (Murray, J., London, 1872).
Todd, E.M. & Kucharski, A. Pain: historical perspectives. in Principles and Practice of Pain Medicine (eds. Warfield, C.A. & Bajwa, Z.H.) 1–10 (McGraw-Hill, 2004).
Melzack, R. & Wall, P.D. Pain mechanisms: a new theory. Science 150, 971–979 (1965).
Baron, R. Neuropathic pain: a clinical perspective. Handb. Exp. Pharmacol. 194, 3–30 (2009).
King, T. et al. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 12, 1364–1366 (2009).
Kurejova, M. et al. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol. Pain 6, 18 (2010).
Woolf, C.J. & Salter, M.W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000).
Ikeda, H., Heinke, B., Ruscheweyh, R. & Sandkuhler, J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299, 1237–1240 (2003).
Toyoda, H. et al. Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex. Mol. Pain 5, 46 (2009).
Woolf, C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688 (1983).
Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772 (2000).
Luo, C., Seeburg, P.H., Sprengel, R. & Kuner, R. Activity-dependent potentiation of calcium signals in spinal sensory networks in inflammatory pain states. Pain 140, 358–367 (2008).
Schoffnegger, D., Ruscheweyh, R. & Sandkuhler, J. Spread of excitation across modality borders in spinal dorsal horn of neuropathic rats. Pain 135, 300–310 (2008).
Ji, R.R., Kohno, T., Moore, K.A. & Woolf, C.J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705 (2003).
Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758 (2009).
Pezet, S. & McMahon, S.B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 29, 507–538 (2006).
Milenkovic, N. et al. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron 56, 893–906 (2007).
Burnashev, N., Monyer, H., Seeburg, P.H. & Sakmann, B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8, 189–198 (1992).
Tong, C.K. & MacDermott, A.B. Both Ca2+-permeable and -impermeable AMPA receptors contribute to primary synaptic drive onto rat dorsal horn neurons. J. Physiol. (Lond.) 575, 133–144 (2006).
Gu, J.G., Albuquerque, C., Lee, C.J. & MacDermott, A.B. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature 381, 793–796 (1996).
Hartmann, B. et al. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron 44, 637–650 (2004).
Xiao, B., Tu, J.C. & Worley, P.F. Homer: a link between neural activity and glutamate receptor function. Curr. Opin. Neurobiol. 10, 370–374 (2000).
Tappe, A. et al. Synaptic scaffolding protein Homer1a protects against chronic inflammatory pain. Nat. Med. 12, 677–681 (2006).
Li, P. et al. AMPA receptor-PDZ interactions in facilitation of spinal sensory synapses. Nat. Neurosci. 2, 972–977 (1999).
Garry, E.M. et al. Specific involvement in neuropathic pain of AMPA receptors and adapter proteins for the GluR2 subunit. Mol. Cell. Neurosci. 24, 10–22 (2003).
Park, J.S. et al. Persistent inflammation induces GluR2 internalization via NMDA receptor–triggered PKC activation in dorsal horn neurons. J. Neurosci. 29, 3206–3219 (2009).
Galan, A., Laird, J.M. & Cervero, F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain 112, 315–323 (2004).
Miletic, G., Dumitrascu, C.I., Honstad, C.E., Micic, D. & Miletic, V. Loose ligation of the rat sciatic nerve elicits early accumulation of Shank1 protein in the post-synaptic density of spinal dorsal horn neurons. Pain 149, 152–159 (2010).
Garry, E.M. et al. Neuropathic sensitization of behavioral reflexes and spinal NMDA receptor/CaM kinase II interactions are disrupted in PSD-95 mutant mice. Curr. Biol. 13, 321–328 (2003).
Dreyer, J. et al. Nitric oxide synthase (NOS)-interacting protein interacts with neuronal NOS and regulates its distribution and activity. J. Neurosci. 24, 10454–10465 (2004).
Wettschureck, N. & Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 (2005).
Willis, W.D. Role of neurotransmitters in sensitization of pain responses. Ann. NY Acad. Sci. 933, 142–156 (2001).
Gold, M.S. & Gebhart, G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. advance online publication, doi:10.1038/nm.2235 (14 October 2010).
Fang, M., Kovacs, K.J., Fisher, L.L. & Larson, A.A. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin. J. Physiol. (Lond.) 549, 903–917 (2003).
Clark, A.K. et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. USA 104, 10655–10660 (2007).
Vergnolle, N. et al. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat. Med. 7, 821–826 (2001).
Narita, M. et al. Protease-activated receptor-1 and platelet-derived growth factor in spinal cord neurons are implicated in neuropathic pain after nerve injury. J. Neurosci. 25, 10000–10009 (2005).
Yamanaka, H. et al. Tissue plasminogen activator in primary afferents induces dorsal horn excitability and pain response after peripheral nerve injury. Eur. J. Neurosci. 19, 93–102 (2004).
Kawasaki, Y. et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 14, 331–336 (2008).
Dai, Y. et al. Proteinase-activated receptor 2–mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J. Neurosci. 24, 4293–4299 (2004).
Clark, A.K., Yip, P.K. & Malcangio, M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J. Neurosci. 29, 6945–6954 (2009).
McMahon, S.B. & Malcangio, M. Current challenges in glia-pain biology. Neuron 64, 46–54 (2009).
Zylka, M.J. et al. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron 60, 111–122 (2008).
Inoue, M. et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 10, 712–718 (2004).
Ji, R.R., Baba, H., Brenner, G.J. & Woolf, C.J. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 2, 1114–1119 (1999).
Hu, H.J. et al. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron 50, 89–100 (2006).
Cheng, H.Y. et al. DREAM is a critical transcriptional repressor for pain modulation. Cell 108, 31–43 (2002).
Derjean, D. et al. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat. Neurosci. 6, 274–281 (2003).
Moore, K.A. et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 22, 6724–6731 (2002).
Scholz, J. et al. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J. Neurosci. 25, 7317–7323 (2005).
Harvey, R.J. et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 (2004).
Coull, J.A. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Coull, J.A. et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003).
Lu, Y., Zheng, J., Xiong, L., Zimmermann, M. & Yang, J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J. Physiol. (Lond.) 586, 5701–5715 (2008).
Jolivalt, C.G., Lee, C.A., Ramos, K.M. & Calcutt, N.A. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain 140, 48–57 (2008).
Knabl, J. et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451, 330–334 (2008).
Schaible, H.G., Ebersberger, A. & Von Banchet, G.S. Mechanisms of pain in arthritis. Ann. NY Acad. Sci. 966, 343–354 (2002).
Abrahamsen, B. et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705 (2008).
Shir, Y. & Seltzer, Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causalgiform pain disorders in rats. Neurosci. Lett. 115, 62–67 (1990).
Seal, R.P. et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655 (2009).
Neumann, S., Doubell, T.P., Leslie, T. & Woolf, C.J. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384, 360–364 (1996).
Bao, L. et al. Peripheral axotomy induces only very limited sprouting of coarse myelinated afferents into inner lamina II of rat spinal cord. Eur. J. Neurosci. 16, 175–185 (2002).
Zhang, Z., Hefferan, M.P. & Loomis, C.W. Topical bicuculline to the rat spinal cord induces highly localized allodynia that is mediated by spinal prostaglandins. Pain 92, 351–361 (2001).
Baba, H. et al. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell. Neurosci. 24, 818–830 (2003).
Torsney, C., Anderson, R.L., Ryce-Paul, K.A. & MacDermott, A.B. Characterization of sensory neuron subpopulations selectively expressing green fluorescent protein in phosphodiesterase 1C BAC transgenic mice. Mol. Pain 2, 17 (2006).
Kolhekar, R., Murphy, S. & Gebhart, G.F. Thalamic NMDA receptors modulate inflammation-produced hyperalgesia in the rat. Pain 71, 31–40 (1997).
Cheong, E. et al. Tuning thalamic firing modes via simultaneous modulation of T- and L-type Ca2+ channels controls pain sensory gating in the thalamus. J. Neurosci. 28, 13331–13340 (2008).
Jeon, D. et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010).
Cao, H. et al. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci. 29, 3307–3321 (2009).
Fu, Y. & Neugebauer, V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J. Neurosci. 28, 3861–3876 (2008).
Neugebauer, V., Li, W., Bird, G.C., Bhave, G. & Gereau, R.W. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J. Neurosci. 23, 52–63 (2003).
Delaney, A.J., Crane, J.W. & Sah, P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 56, 880–892 (2007).
Eippert, F. et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543 (2009).
Porreca, F., Ossipov, M.H. & Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 25, 319–325 (2002).
Heinricher, M.M., Tavares, I., Leith, J.L. & Lumb, B.M. Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev. 60, 214–225 (2009).
Zhao, Z.Q. et al. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J. Neurosci. 27, 6045–6053 (2007).
Zhao, Z.Q. et al. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc. Natl. Acad. Sci. USA 104, 14519–14524 (2007).
Zhang, W. et al. Neuropathic pain is maintained by brainstem neurons co-expressing opioid and cholecystokinin receptors. Brain 132, 778–787 (2009).
Wei, F., Guo, W., Zou, S., Ren, K. & Dubner, R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J. Neurosci. 28, 10482–10495 (2008).
Guo, W. et al. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J. Neurosci. 26, 126–137 (2006).
Suzuki, R., Morcuende, S., Webber, M., Hunt, S.P. & Dickenson, A.H. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat. Neurosci. 5, 1319–1326 (2002).
Fields, H.L. Pain modulation: expectation, opioid analgesia and virtual pain. Prog. Brain Res. 122, 245–253 (2000).
Edelmayer, R.M. et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann. Neurol. 65, 184–193 (2009).
Li, P. & Zhuo, M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature 393, 695–698 (1998).
Wei, F. et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 30, 8624–8636 (2010).
Seminowicz, D.A. et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology 139, 48–57 (2010).
May, A. Morphing voxels: the hype around structural imaging of headache patients. Brain 132, 1419–1425 (2009).
Rodriguez-Raecke, R., Niemeier, A., Ihle, K., Ruether, W. & May, A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J. Neurosci. 29, 13746–13750 (2009).
Seminowicz, D.A. et al. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage 47, 1007–1014 (2009).
Schweizerhof, M. et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat. Med. 15, 802–807 (2009).
Ceyhan, G.O. et al. Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology 136, 177–186 (2009).
Siau, C., Xiao, W. & Bennett, G.J. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp. Neurol. 201, 507–514 (2006).
Cain, D.M. et al. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J. Neurosci. 21, 9367–9376 (2001).
Hofer, S.B. & Bonhoeffer, T. Dendritic spines: the stuff that memories are made of? Curr. Biol. 20, R157–R159 (2010).
Saneyoshi, T., Fortin, D.A. & Soderling, T.R. Regulation of spine and synapse formation by activity-dependent intracellular signaling pathways. Curr. Opin. Neurobiol. 20, 108–115 (2010).
Yang, G., Pan, F. & Gan, W.B. Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924 (2009).
Tan, A.M. et al. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J. Neurosci. 28, 13173–13183 (2008).
Hotulainen, P. & Hoogenraad, C.C. Actin in dendritic spines: connecting dynamics to function. J. Cell Biol. 189, 619–629 (2010).
Luo, L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1, 173–180 (2000).
Eroglu, C. et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 (2009).
Acknowledgements
I thank R. Lefaucheur for secretarial assistance and members of the laboratory for discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Kuner, R. Central mechanisms of pathological pain. Nat Med 16, 1258–1266 (2010). https://doi.org/10.1038/nm.2231
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2231
This article is cited by
-
Intraarticular monomethyl fumarate as a perspective therapy for osteoarthritis by macrophage polarization
Inflammopharmacology (2024)
-
Effect of high-frequency repetitive transcranial magnetic stimulation under different intensities upon rehabilitation of chronic pelvic pain syndrome: protocol for a randomized controlled trial
Trials (2023)
-
Activation of CB1R alleviates central sensitization by regulating HCN2-pNR2B signaling in a chronic migraine rat model
The Journal of Headache and Pain (2023)
-
Machine learning analysis predicts a person’s sex based on mechanical but not thermal pain thresholds
Scientific Reports (2023)
-
Synchronized activity of sensory neurons initiates cortical synchrony in a model of neuropathic pain
Nature Communications (2023)