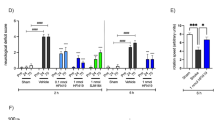
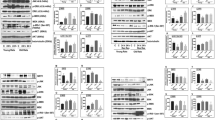
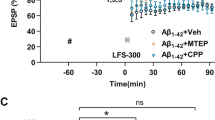
Abstract
Excitotoxic neuronal damage caused by overactivation of N-methyl-D-aspartate glutamate receptors (NMDARs) is thought to be a principal cause of neuronal loss after stroke and brain trauma. Here we report that activation of sterol regulatory element binding protein-1 (SREBP-1) transcription factor in affected neurons is an essential step in NMDAR-mediated excitotoxic neuronal death in both in vitro and in vivo models of stroke. The NMDAR-mediated activation of SREBP-1 is a result of increased insulin-induced gene-1 (Insig-1) degradation, which can be inhibited with an Insig-1–derived interference peptide (Indip) that we have developed. Using a focal ischemia model of stroke, we show that systemic administration of Indip not only prevents SREBP-1 activation but also substantially reduces neuronal damage and improves behavioral outcome. Our study suggests that agents that reduce SREBP-1 activation such as Indip may represent a new class of neuroprotective therapeutics against stroke.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Choi, D.W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 11, 465–469 (1988).
Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 5, 160–170 (2006).
Ikonomidou, C. & Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 1, 383–386 (2002).
Kemp, J.A. & McKernan, R.M. NMDA receptor pathways as drug targets. Nat. Neurosci. 5 (Suppl), 1039–1042 (2002).
Lee, J.M., Zipfel, G.J. & Choi, D.W. The changing landscape of ischaemic brain injury mechanisms. Nature 399, A7–A14 (1999).
Aarts, M. et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 298, 846–850 (2002).
Camandola, S. & Mattson, M.P. NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opin. Ther. Targets 11, 123–132 (2007).
Hardingham, G.E., Arnold, F.J. & Bading, H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat. Neurosci. 4, 261–267 (2001).
Hetman, M. & Kharebava, G. Survival signaling pathways activated by NMDA receptors. Curr. Top. Med. Chem. 6, 787–799 (2006).
Rao, V.R. & Finkbeiner, S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 30, 284–291 (2007).
West, A.E., Griffith, E.C. & Greenberg, M.E. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 3, 921–931 (2002).
Zhang, S.J. et al. Decoding NMDA receptor signaling: identification of genomic programs specifying neuronal survival and death. Neuron 53, 549–562 (2007).
Zou, J. & Crews, F. CREB and NF-κB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cell. Mol. Neurobiol. 26, 385–405 (2006).
Espenshade, P.J. & Hughes, A.L. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 41, 401–427 (2007).
Goldstein, J.L., DeBose-Boyd, R.A. & Brown, M.S. Protein sensors for membrane sterols. Cell 124, 35–46 (2006).
Adibhatla, R.M., Hatcher, J.F. & Dempsey, R.J. Lipids and lipidomics in brain injury and diseases. AAPS J. 8, E314–E321 (2006).
Siesjö, B.K. & Katsura, K. Ischemic brain damage: focus on lipids and lipid mediators. Adv. Exp. Med. Biol. 318, 41–56 (1992).
Sandberg, M.B. et al. Glucose-induced lipogenesis in pancreatic beta cells is dependent on SREBP-1. Mol. Cell. Endocrinol. 240, 94–106 (2005).
Takahashi, A. et al. Transgenic mice overexpressing nuclear SREBP-1c in pancreatic beta cells. Diabetes 54, 492–499 (2005).
Wang, H. et al. The transcription factor SREBP-1c is instrumental in the development of betacell dysfunction. J. Biol. Chem. 278, 16622–16629 (2003).
Wang, H., Kouri, G. & Wollheim, C.B. ER stress and SREBP-1 activation are implicated in beta cell glucolipotoxicity. J. Cell Sci. 118, 3905–3915 (2005).
Yamashita, T. et al. Role of uncoupling protein-2 up-regulation and triglyceride accumulation in impaired glucose-stimulated insulin secretion in a beta cell lipotoxicity model overexpressing sterol regulatory element–binding protein-1c. Endocrinology 145, 3566–3577 (2004).
Chang, Y.C., Bien, C.M., Lee, H., Espenshade, P.J. & Kwon-Chung, K.J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 64, 614–629 (2007).
Todd, B.L., Stewart, E.V., Burg, J.S., Hughes, A.L. & Espenshade, P.J. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol. Cell. Biol. 26, 2817–2831 (2006).
Taghibiglou, C. et al. Essential role of SBP-1 activation in oxygen deprivation induced lipid accumulation and increase in body width/length ratio in Caenorhabditis elegans. FEBS Lett. 583, 831–834 (2009).
Hardingham, G.E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Liu, Y. et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 27, 2846–2857 (2007).
Zhou, M. & Baudry, M. Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J. Neurosci. 26, 2956–2963 (2006).
Liu, L. et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304, 1021–1024 (2004).
Tigaret, C.M. et al. Subunit dependencies of N-methyl-d-aspartate (NMDA) receptor–induced α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization. Mol. Pharmacol. 69, 1251–1259 (2006).
Mutel, V. et al. In vitro binding properties in rat brain of [3H]Ro 25–6981, a potent and selective antagonist of NMDA receptors containing NR2B subunits. J. Neurochem. 70, 2147–2155 (1998).
Mattson, M.P. Calcium and neurodegeneration. Aging Cell 6, 337–350 (2007).
Wang, X. et al. Cleavage of sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 15, 1012–1020 (1996).
Horton, J.D. et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA 100, 12027–12032 (2003).
Horton, J.D. et al. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element–binding protein-2. J. Clin. Invest. 101, 2331–2339 (1998).
Nohturfft, A., Brown, M.S. & Goldstein, J.L. Topology of SREBP cleavage-activating protein, a polytopic membrane protein with a sterol-sensing domain. J. Biol. Chem. 273, 17243–17250 (1998).
Sun, L.P., Li, L., Goldstein, J.L. & Brown, M.S. Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J. Biol. Chem. 280, 26483–26490 (2005).
Yang, T. et al. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell 110, 489–500 (2002).
Gong, Y. et al. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 3, 15–24 (2006).
Lee, J.N., Gong, Y., Zhang, X. & Ye, J. Proteasomal degradation of ubiquitinated Insig proteins is determined by serine residues flanking ubiquitinated lysines. Proc. Natl. Acad. Sci. USA 103, 4958–4963 (2006).
Lee, J.N., Song, B., DeBose-Boyd, R.A. & Ye, J. Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J. Biol. Chem. 281, 39308–39315 (2006).
Lee, J.N. & Ye, J. Proteolytic activation of sterol regulatory element–binding protein induced by cellular stress through depletion of Insig-1. J. Biol. Chem. 279, 45257–45265 (2004).
Schwarze, S.R., Ho, A., Vocero-Akbani, A. & Dowdy, S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Borsello, T. et al. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9, 1180–1186 (2003).
Liu, X.J. et al. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat. Med. 14, 1325–1332 (2008).
Wong, T.P. et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc. Natl. Acad. Sci. USA 104, 11471–11476 (2007).
Goldberg, M.P. & Choi, D.W. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 13, 3510–3524 (1993).
Bederson, J.B. et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17, 472–476 (1986).
Schmued, L.C., Albertson, C. & Slikker, W. Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 751, 37–46 (1997).
Park, H.J. et al. Role of sterol regulatory element binding proteins in the regulation of Gαi2 expression in cultured atrial cells. Circ. Res. 91, 32–37 (2002).
Park, H.J. et al. Parasympathetic response in chick myocytes and mouse heart is controlled by SREBP. J. Clin. Invest. 118, 259–271 (2008).
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008).
Lee, S.H. et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319, 1253–1256 (2008).
Mielke, J.G. & Wang, Y.T. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. J. Neurochem. 92, 103–113 (2005).
Acknowledgements
We thank J. Ye (University of Texas Southwestern Medical Center) for myc–Insig-1 plasmid, T. Osborne (University of California–Irvine) for Flag–nt-SREBP-1 and Y. P. Auberson (Novartis Pharma AG) for the generous gift of NVP-AAM077. We also thank J. Wang for his technical support. This work was supported by the Heart and Stroke Foundation of British Columbia and the Yukon, the Canadian Institutes of Health Research and CHDI (Cure Huntington's Disease Initiative) Foundation. Y.T.W. is a Howard Hughes Medical Institute International Scholar and Heart and Stroke Foundation of British Columbia and the Yukon Chair in Stroke Research. C.T. was supported by post-doctoral fellowships from the Canadian Institutes of Health Research and Michael Smith Foundation for Health.
Author information
Authors and Affiliations
Contributions
C.T. initiated the study, wrote the manuscript and performed all biochemical experiments, H.G.S.M. performed most cell biology experiments and helped write the manuscript, T.W.L. performed the in vivo studies, T.C. and S.Z. contributed to the fluorescent microscopy, S.P., L.K. and Y.H.W. performed mRNA analysis and transcription factor screen, Y.L. aided in the in vivo studies, J.L. and J.Z.Z.W. designed and made Insig-1–specific antibody, E.L. aided in manuscript preparation, Y.P.L. and J.-H.I. prepared neuronal cultures and developed transfection methods, M.S.C. cosupervised the experiments of mRNA analysis transcription factor screen, and Y.T.W. designed the study and supervised the overall project.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 2150 kb)
Rights and permissions
About this article
Cite this article
Taghibiglou, C., Martin, H., Lai, T. et al. Role of NMDA receptor–dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nat Med 15, 1399–1406 (2009). https://doi.org/10.1038/nm.2064
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2064
This article is cited by
-
Cerebrolysin reduces excitotoxicity by modulation of cell-death proteins in delayed hours of ischemic reperfusion injury
Metabolic Brain Disease (2023)
-
Mice and Rats Exhibit Striking Inter-species Differences in Gene Response to Acute Stroke
Cellular and Molecular Neurobiology (2022)
-
NMMHC IIA triggered lipid metabolize reprogramming resulting in vascular endothelial cellular tight junction injury
Molecular Biology Reports (2022)
-
Targeting NMDA receptors in stroke: new hope in neuroprotection
Molecular Brain (2018)
-
Discovery and development of NA-1 for the treatment of acute ischemic stroke
Acta Pharmacologica Sinica (2018)