Abstract
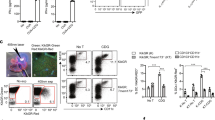
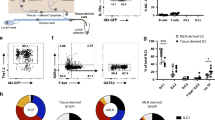
We report that infection of draining lymph nodes (DLNs) by Salmonella typhimurium results in the specific downregulation of the homeostatic chemokines CCL21 and CXCL13, which are essential for normal DLN organization and function. Our data reveal that the mechanism of this suppression is dependent on S. typhimurium LPS (sLPS). The decrease in CCL21 expression involves interaction between sLPS and CCL21-producing cells within DLNs, triggering a distinct Toll-like receptor 4 (TLR4)-mediated host signaling response. In this response, suppressor of cytokine signaling-3 (Socs3) is upregulated, which negatively regulates mothers against decapentaplegic homolog-3 (Smad3)-initiated production of CCL21. Disruption of lymph node architecture and cellular trafficking enhances S. typhimurium virulence and could represent a mechanism of immune suppression used by pathogens that primarily target lymphoid tissue.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gunn, M.D. et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA 95, 258–263 (1998).
Gunn, M.D. et al. A B cell–homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Cyster, J.G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Cyster, J.G. et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176, 181–193 (2000).
Ansel, K.M., Harris, R.B. & Cyster, J.G. CXCL13 is required for B1 cell homing, natural antibody production and body cavity immunity. Immunity 16, 67–76 (2002).
Mori, S. et al. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 193, 207–218 (2001).
Oyston, P.C., Sjostedt, A. & Titball, R.W. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2, 967–978 (2004).
Bonneau, M. et al. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J. Leukoc. Biol. 79, 268–276 (2006).
Perry, R.D. & Fetherston, J.D. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10, 35–66 (1997).
Jones, B.D. & Falkow, S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14, 533–561 (1996).
Mittrücker, H.W., Raupach, B., Kohler, A. & Kaufmann, S.H. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J. Immunol. 164, 1648–1652 (2000).
Kumar, A., Kumar, R., Malaviya, A.N. & Mohapatra, L.N. Mouse typhoid in normal & T-cell deficient animals. Indian J. Med. Res. 80, 557–562 (1984).
Kumar, A., Kumar, R., Malaviya, A.N. & Mohapatra, L.N. Typhoid in normal & B-cell deficient mice. Indian J. Med. Res. 80, 270–278 (1984).
Nauciel, C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J. Immunol. 145, 1265–1269 (1990).
Somerville, J.E. Jr., Cassiano, L., Bainbridge, B., Cunningham, M.D. & Darveau, R.P. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Invest. 97, 359–365 (1996).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Khan, S.A. et al. A lethal role for lipid A in Salmonella infections. Mol. Microbiol. 29, 571–579 (1998).
Low, K.B. et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat. Biotechnol. 17, 37–41 (1999).
O'Connell, K.A. & Edidin, M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J. Immunol. 144, 521–525 (1990).
Mueller, S.N. et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317, 670–674 (2007).
Uchiya, K. & Nikai, T. Salmonella pathogenicity island 2–dependent expression of suppressor of cytokine signaling 3 in macrophages. Infect. Immun. 73, 5587–5594 (2005).
Stoiber, D. et al. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-γa. J. Immunol. 163, 2640–2647 (1999).
Liu, X., Zhang, Y., Yu, Y., Yang, X. & Cao, X. SOCS3 promotes TLR4 response in macrophages by feedback inhibiting TGF-β1/Smad3 signaling. Mol. Immunol. 45, 1405–1413 (2008).
Acknowledgements
We would like to thank M. Krangel, M. Gunn, M. Kuehn, Y. He and W. Zhang for their discussions and J. Wright for the use of equipment. Salmonella strains χ3761 and χ8573 were a gift from R. Curtiss (Arizona State University) and M. Kuehn (Duke University). S. typhimurium SL1344 was a gift from A. Aballay (Duke University), and E. coli J96 was a gift from S. Normark (Umea University). G. Li provided technical advice. Z. Swan, C. Kunder, G. Li and A. Bickell provided critical manuscript review. This work was supported by US National Institutes of Health grants R01 AI35678, R01 DK077159, R01 AI50021, R37 DK50814 and R21 AI056101.
Author information
Authors and Affiliations
Contributions
All experiments were performed by A.L.S. Experiments were designed by A.L.S. and S.N.A.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–13, Supplementary Table 1 and Supplementary Methods (PDF 892 kb)
Rights and permissions
About this article
Cite this article
St John, A., Abraham, S. Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat Med 15, 1259–1265 (2009). https://doi.org/10.1038/nm.2036
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2036
This article is cited by
-
Nature and consequences of interactions between Salmonella enterica serovar Dublin and host cells in cattle
Veterinary Research (2019)
-
Mass spectrometry imaging identifies palmitoylcarnitine as an immunological mediator during Salmonella Typhimurium infection
Scientific Reports (2017)
-
Potential immunosuppressive effects of Escherichia coli O157:H7 experimental infection on the bovine host
BMC Genomics (2016)
-
Tumor-induced stromal reprogramming drives lymph node transformation
Nature Immunology (2016)
-
SOCS3 revisited: a broad regulator of disease, now ready for therapeutic use?
Cellular and Molecular Life Sciences (2016)