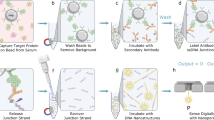
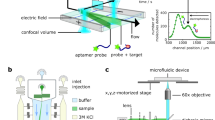
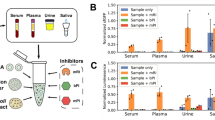
Abstract
Advances in biosensor technologies for in vitro diagnostics have the potential to transform the practice of medicine. Despite considerable work in the biosensor field, there is still no general sensing platform that can be ubiquitously applied to detect the constellation of biomolecules in diverse clinical samples (for example, serum, urine, cell lysates or saliva) with high sensitivity and large linear dynamic range. A major limitation confounding other technologies is signal distortion that occurs in various matrices due to heterogeneity in ionic strength, pH, temperature and autofluorescence. Here we present a magnetic nanosensor technology that is matrix insensitive yet still capable of rapid, multiplex protein detection with resolution down to attomolar concentrations and extensive linear dynamic range. The matrix insensitivity of our platform to various media demonstrates that our magnetic nanosensor technology can be directly applied to a variety of settings such as molecular biology, clinical diagnostics and biodefense.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Srinivas, P.R. et al. Proteomics in early detection of cancer. Clin. Chem. 47, 1901–1911 (2001).
Lopez, M.F. et al. A novel, high-throughput workflow for discovery and identification of serum carrier protein-bound peptide biomarker candidates in ovarian cancer samples. Clin. Chem. 53, 1067–1074 (2007).
Gorelik, E. et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 14, 981–987 (2005).
Zheng, Y. et al. A multiparametric panel for ovarian cancer diagnosis, prognosis, and response to chemotherapy. Clin. Cancer Res. 13, 6984–6992 (2007).
Heath, J.R. & Davis, M.E. Nanotechnology and cancer. Annu. Rev. Med. 59, 251–265 (2008).
Mitchell, P. A perspective on protein microarrays. Nat. Biotechnol. 20, 225–229 (2002).
Chan, S.M. et al. Protein microarrays for multiplex analysis of signal transduction pathways. Nat. Med. 10, 1390–1396 (2004).
Shingyoji, M. et al. Quantum dots–based reverse phase protein microarray. Talanta 67, 472–478 (2005).
Zheng, G. et al. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 23, 1294–1301 (2005).
Ji, H. et al. Microcantilever biosensors based on conformational change of proteins. Analyst 133, 434–443 (2008).
Ghosh, S., Sood, A.K. & Kumar, N. Carbon nanotube flow sensors. Science 299, 1042–1044 (2003).
Drummond, T.G., Hill, M.G. & Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 21, 1192–1199 (2003).
Cheng, M.M. et al. Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 10, 11–19 (2006).
Barnas, J. et al. Novel magnetoresistance effect in layered magnetic structures: Theory and experiment. Phys. Rev. B Condens. Matter 42, 8110–8120 (1990).
Prinz, G.A. Magnetoelectronics. Science 282, 1660–1663 (1998).
Wolf, S.A. et al. Spintronics: A spin-based electronics vision for the future. Science 294, 1488–1495 (2001).
Baselt, D.R. et al. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 13, 731–739 (1998).
Li, G. et al. Detection of single micron-sized magnetic bead and magnetic nanoparticles using spin valve sensors for biological applications. J. Appl. Phys. 93, 7557–7559 (2003).
Graham, D.L. et al. Single magnetic microsphere placement and detection on-chip using current line designs with integrated spin valve sensors: biotechnological applications. J. Appl. Phys. 91, 7786–7788 (2002).
Schotter, J. et al. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosens. Bioelectron. 19, 1149–1156 (2004).
Millen, R.L. et al. Giant magnetoresistive sensors and superparamagnetic nanoparticles: a chip-scale detection strategy for immunosorbent assays. Anal. Chem. 77, 6581–6587 (2005).
Li, G. et al. Spin valve sensors for ultrasensitive detection of superparamagnetic nanoparticles for biological applications. Sens. Actuators A Phys. 126, 98–106 (2006).
Xu, L. et al. Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens. Bioelectron. 24, 99–103 (2008).
Osterfeld, S.J. et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 105, 20637–20640 (2008).
Arao, S. et al. Measurement of urinary lactoferrin as a marker of urinary tract infection. J. Clin. Microbiol. 37, 553–557 (1999).
Stern, E. et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 445, 519–522 (2007).
Georganopoulou, D.G. et al. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer's disease. Proc. Natl. Acad. Sci. USA 102, 2273–2276 (2005).
Wu, G. et al. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nat. Biotechnol. 19, 856–860 (2001).
Zimmerman, R., Wahren, B. & Edsmyr, F. Assessment of serial CEA determinations in urine of patients with bladder carcinoma. Cancer 46, 1802–1809 (1980).
Mukherjee, S. et al. A longitudinal study of unsaturated iron-binding capacity and lactoferrin in unstimulated parotid saliva. Biol. Trace Elem. Res. 57, 1–8 (1997).
Chen, Z. et al. Protein microarrays with carbon nanotubes as multicolor Raman labels. Nat. Biotechnol. 26, 1285–1292 (2008).
Cui, Y. et al. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 293, 1289–1292 (2001).
Tom, B.H. et al. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro 12, 180–191 (1976).
Acknowledgements
This work was supported in part by US National Cancer Institute grants 1U54CA119367 and N44CM–2009-00011, US National Science Foundation grant ECCS-0801385-000, US Defense Threat Reduction Agency grant HDTRA1-07-1-0030-P00005, the US Defense Advanced Research Projects Agency/Navy Grant N00014–02-1–0807, NCI ICMIC P50 CA114747, the US Department of Veterans Affairs Merit Review B4872, the Canary Foundation and The National Semiconductor Corporation. R.S.G. acknowledges financial support from Stanford Medical School Medical Scientist Training Program and a National Science Foundation graduate research fellowship. C.H.N. acknowledges financial support from the Denmark-American Foundation and the Lundbeck Foundation.
Author information
Authors and Affiliations
Contributions
R.S.G., D.A.H., S.S.G. and S.X.W designed research; R.S.G., D.A.H., C.H.N. and K.E.M. performed research; R.S.G., D.A.H., C.H.N., S.J.O., H.Y., K.E.M., R.J.W., B.M., J.C.L., S.S.G. and S.X.W contributed new reagents and/or analytical tools; R.S.G., D.A.H. and S.X.W analyzed data; S.J.O. and S.X.W. designed the magnetic sensors; R.S.G. and H.Y. developed the biochemistry; and R.S.G., S.S.G. and S.X.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
Stanford University has licensed part of the magnetic bioassay chip technology contained in this publication to MagArray Inc., an early-stage startup company in Silicon Valley, California. S.X.W., S.S.G., H.Y. and S.J.O. hold financial interests in MagArray in the form of stock options.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–9 (PDF 928 kb)
Rights and permissions
About this article
Cite this article
Gaster, R., Hall, D., Nielsen, C. et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat Med 15, 1327–1332 (2009). https://doi.org/10.1038/nm.2032
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2032
This article is cited by
-
Quantitative and rapid detection of morphine and hydromorphone at the point of care by an automated giant magnetoresistive nanosensor platform
Analytical and Bioanalytical Chemistry (2022)
-
Magnetic supercluster particles for highly sensitive magnetic biosensing of proteins
Microchimica Acta (2022)
-
Reagentless biomolecular analysis using a molecular pendulum
Nature Chemistry (2021)
-
Reusable surface amplified nanobiosensor for the sub PFU/mL level detection of airborne virus
Scientific Reports (2021)
-
Giant magnetoresistive biosensors for real-time quantitative detection of protease activity
Scientific Reports (2020)