Abstract
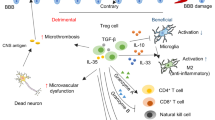
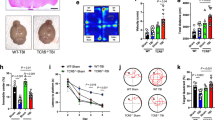
Lymphocyte recruitment and activation have been implicated in the progression of cerebral ischemia-reperfusion (I/R) injury, but the roles of specific lymphocyte subpopulations and cytokines during stroke remain to be clarified. Here we demonstrate that the infiltration of T cells into the brain, as well as the cytokines interleukin-23 (IL-23) and IL-17, have pivotal roles in the evolution of brain infarction and accompanying neurological deficits. Blockade of T cell infiltration into the brain by the immunosuppressant FTY720 reduced I/R-induced brain damage. The expression of IL-23, which was derived mostly from infiltrated macrophages, increased on day 1 after I/R, whereas IL-17 levels were elevated after day 3, and this induction of IL-17 was dependent on IL-23. These data, together with analysis of mice genetically disrupted for IL-17 and IL-23, suggest that IL-23 functions in the immediate stage of I/R brain injury, whereas IL-17 has an important role in the delayed phase of I/R injury during which apoptotic neuronal death occurs in the penumbra. Intracellular cytokine staining revealed that γδT lymphocytes, but not CD4+ helper T cells, were a major source of IL-17. Moreover, depletion of γδT lymphocytes ameliorated the I/R injury. We propose that T lymphocytes, including γδT lymphocytes, could be a therapeutic target for mitigating the inflammatory events that amplify the initial damage in cerebral ischemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sacco, R.L., Chong, J.Y., Prabhakaran, S. & Elkind, M.S. Experimental treatments for acute ischaemic stroke. Lancet 369, 331–341 (2007).
Ooboshi, H. et al. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 111, 913–919 (2006).
Schroeter, M., Jander, S., Witte, O.W. & Stoll, G. Local immune responses in the rat middle cerebral artery occlusion. J. Neuroimmunol. 55, 195–203 (1994).
Jander, S., Karemer, M., Schroeter, M., Witte, O.W. & Stoll, G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J. Cereb. Blood Flow Metab. 15, 42–51 (1995).
Yilmaz, G., Arumugam, T.V., Stokes, K.Y. & Granger, D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113, 2105–2112 (2006).
Lambertsen, K.L. et al. A role for interferon-gamma in focal cerebral ischemia in mice. J. Neuropathol. Exp. Neurol. 63, 942–955 (2004).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508 (2002).
Sutton, C., Brereton, C., Keogh, B., Mills, K.H. & Lavelle, E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Chen, Y. et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326 (2006).
Kebir, H. et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 13, 1173–1175 (2007).
Ifergan, I. et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain 131, 785–799 (2008).
Li, G.Z. et al. Expression of interleukin-17 in ischemic brain tissue. Scand. J. Immunol. 62, 481–486 (2005).
Charriaut-Marlangue, C. et al. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J. Cereb. Blood Flow Metab. 16, 186–194 (1996).
Matsui, T. et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: enhanced astrocytic synthesis of s-100beta in the periinfarct area precedes delayed infarct expansion. J. Cereb. Blood Flow Metab. 22, 711–722 (2002).
Campanella, M., Sciorati, C., Tarazzo, G. & Beltramo, M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke 33, 586–592 (2002).
Yamasaki, Y. et al. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 26, 676–680 (1995).
Romanic, A.M., White, R.F., Arleth, A.J., Ohlstein, E.H. & Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29, 1020–1030 (1998).
Yong, V.W., Power, C., Forsyth, P. & Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2, 502–511 (2001).
Fujimoto, M. et al. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 28, 1674–1685 (2008).
Friedlander, R.M. et al. Expression of a dominant negative mutant of interleukin-1 beta converting enzyme in transgenic mice prevents neuronal cell death induced by trophic factor withdrawal and ischemic brain injury. J. Exp. Med. 185, 933–940 (1997).
Shibata, K., Yamada, H., Hara, H., Kishihara, K. & Yoshikai, Y. Resident Vδ1+γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466–4472 (2007).
Jensen, K.D. et al. Thymic selection determines gammadelta T cell effector fate: antigen-naïve cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity 29, 90–100 (2008).
Nakamura, R. et al. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J. Immunol. 181, 2071–2075 (2008).
Gaudinski, M.R. et al. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke 39, 2765–2768 (2008).
Shimosegawa, E. et al. Metabolic penumbra of acute brain infarction: a correlation with infarct growth. Ann. Neurol. 57, 495–504 (2005).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Tanaka, K. et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J. Immunol. 180, 3746–3756 (2008).
Hara, H. et al. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. USA 94, 2007–2012 (1997).
Sugimori, H. et al. Krypton laser-induced photothrombotic distal middle cerebral artery occlusion without craniectomy in mice. Brain Res. Brain Res. Protoc. 13, 189–196 (2004).
Acknowledgements
We thank S. Hamano for TCRγδ-specific antibody hybridoma, T. Yoshioka, N. Shiino and M. Asakawa for technical assistance, E. Ishikawa and H. Noguchi for technical support for human ischemic brain section, T. Kobayashi for discussion and technical help and N. Soma for manuscript preparation. This study was supported by special Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO, 07-4), the Naito Foundation and the Astellas Foundation for Research on Metabolic Disorders.
Author information
Authors and Affiliations
Contributions
T.S. designed and performed experiments, analyzed data and wrote the manuscript; Y.S. performed experiments and analyzed data; H.O. contributed to the experimental design, analysis and manuscript writing; H.S. performed experiments; R.N. provided specific input into flow cytometry and mouse analysis, I.T. contributed to manuscript writing; T.I. and Y.O. contributed to histochemistry; M.I. contributed to the experimental design; D.J.C. and Y.I. provided mice and critical input on IL-23 and IL-17 functions; A.Y. initiated and directed the entire study, designed experiments and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–7, Supplementary Tables 1–4 and Supplementary Methods (PDF 564 kb)
Rights and permissions
About this article
Cite this article
Shichita, T., Sugiyama, Y., Ooboshi, H. et al. Pivotal role of cerebral interleukin-17–producing γδT cells in the delayed phase of ischemic brain injury. Nat Med 15, 946–950 (2009). https://doi.org/10.1038/nm.1999
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1999
This article is cited by
-
Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke
Journal of Neuroinflammation (2024)
-
Exaggerated levels of some specific TLRs, cytokines and chemokines in Japanese encephalitis infected BV2 and neuro 2A cell lines associated with worst outcome
Virology Journal (2023)
-
Delayed NLRP3 inflammasome inhibition ameliorates subacute stroke progression in mice
Journal of Neuroinflammation (2023)
-
IL-17A promotes the progression of Alzheimer’s disease in APP/PS1 mice
Immunity & Ageing (2023)
-
Vγ1 and Vγ4 gamma-delta T cells play opposing roles in the immunopathology of traumatic brain injury in males
Nature Communications (2023)