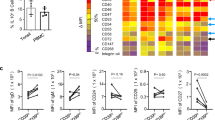
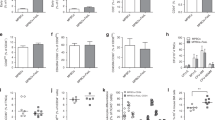
Abstract
Memory T cells promote allograft rejection particularly in co-stimulation blockade–based immunosuppressive regimens. Here we show that the CD2-specific fusion protein alefacept (lymphocyte function–associated antigen-3–Ig; LFA -3–Ig) selectively eliminates memory T cells and, when combined with a co-stimulation blockade–based regimen using cytotoxic T lymphocyte antigen-4 (CTLA-4)-Ig, a CD80- and CD86-specific fusion protein, prevents renal allograft rejection and alloantibody formation in nonhuman primates. These results support the immediate translation of a regimen for the prevention of allograft rejection without the use of calcineurin inhibitors, steroids or pan–T cell depletion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Meier-Kriesche, H.U. et al. Am. J. Transplant. 6, 1111–1131 (2006).
Lin, H. et al. J. Exp. Med. 178, 1801–1806 (1993).
Li, Y., Zheng, X.X., Li, X.C., Zand, M.S. & Strom, T.B. Transplantation 66, 1387–1388 (1998).
Kirk, A.D. et al. Immunol. Rev. 196, 176–196 (2003).
Valujskikh, A. & Li, X.C. J. Am. Soc. Nephrol. 18, 2252–2261 (2007).
Adams, A.B. et al. J. Clin. Invest. 111, 1887–1895 (2003).
Wu, Z. et al. Nat. Med. 10, 87–92 (2004).
Ortonne, J.P., Lebwohl, M., Em Griffiths, C. & Alefacept Clinical Study Group Eur. J. Dermatol. 13, 117–123 (2003).
Ellis, C.N., Krueger, G.G. & Alefacept Clinical Study Group N. Engl. J. Med. 345, 248–255 (2001).
Chamian, F. et al. Proc. Natl. Acad. Sci. USA 102, 2075–2080 (2005).
Pitcher, C.J. et al. J. Immunol. 168, 29–43 (2002).
Preston, E.H. et al. Am. J. Transplant. 5, 1032–1041 (2005).
Girlanda, R. et al. Am. J. Transplant. 8, 600–607 (2008).
Harari, A. et al. Immunol. Rev. 211, 236–254 (2006).
Acknowledgements
This study was funded in part by the Division of Intramural Research, National Institute of Diabetes, Digestive and Kidney Disease, NIH (Z01 DK062007-06). Salary support for T.A.W. was provided by the Howard Hughes Medical Institute through the NIH Research Scholars Program. A.D.K. is supported by the National Institutes of Health (1U01AI079223-01A1), the Georgia Research Alliance and the McKelvey Foundation. We gratefully acknowledge the expert assistance of J. Bacher and the staff of the NIH Veterinary Pathology Department.
Author information
Authors and Affiliations
Contributions
T.A.W. performed surgical procedures, cared for experimental macaques, conducted in vitro experiments, interpreted data and prepared the manuscript; A.H.C. performed surgical procedures and cared for experimental macaques; A.A. performed surgical procedures, cared for experimental macaques, conducted in vitro experiments and interpreted data; A.P.T. performed surgical procedures, cared for experimental macaques, conducted in vitro experiments and interpreted data; M.R. cared for experimental macaques, conducted in vitro experiments and interpreted data; F.V.L. performed surgical procedures and cared for experimental macaques; R.L.K. conducted in vitro experiments and interpreted data; L.S. conducted in vitro experiments and interpreted data;, M.S. performed histology and immunohistochemistry, interpreted data and prepared the manuscript; C.P.L. interpreted data and prepared the manuscript; A.D.K. conceived of experimental design, performed surgical procedures, cared for experimental macaques, interpreted data and prepared the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figs. 1–4 and Supplementary Methods (PDF 6015 kb)
Rights and permissions
About this article
Cite this article
Weaver, T., Charafeddine, A., Agarwal, A. et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med 15, 746–749 (2009). https://doi.org/10.1038/nm.1993
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1993
This article is cited by
-
Antigen discrimination by T cells relies on size-constrained microvillar contact
Nature Communications (2023)
-
Activation and regulation of alloreactive T cell immunity in solid organ transplantation
Nature Reviews Nephrology (2022)
-
High-Dimensional Renal Profiling: Towards a Better Understanding of Renal Transplant Immune Suppression
Current Transplantation Reports (2019)
-
Allogeneic stem cell transplantation in fully MHC-matched Mauritian cynomolgus macaques recapitulates diverse human clinical outcomes
Nature Communications (2017)
-
Memory T cells in organ transplantation: progress and challenges
Nature Reviews Nephrology (2016)