Abstract
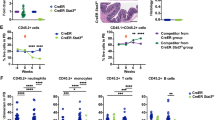
Type I interferons (IFNs), a family of cytokines, orchestrate numerous biological and cellular processes1,2,3. Although it is well known that type I IFNs are essential for establishing the host antiviral state4, their role in hematopoietic homeostasis has not been studied. Here we show that type I IFNs induce proliferation and exhaustion in hematopoietic stem cells (HSCs) and that interferon regulatory factor-2 (IRF2), a transcriptional suppressor of type I IFN signaling5,6, preserves the self-renewal and multilineage differentiation capacity of HSCs. HSCs were substantially less abundant in the bone marrow of Irf2−/− as compared to Irf2+/− mice. Irf2−/− HSCs showed enhanced cell cycling status and failed to produce hematopoietic cells in competitive repopulation assays, and the reconstituting capacity of Irf2−/− HSCs was restored by disabling type I IFN signaling in these cells. In wild-type mice, injection of poly(I:C), an inducer of type I IFN signaling, or IFN-α induced HSC proliferation, and chronic type I IFN signaling further reduced the number of quiescent HSCs. Notably, combined poly(I:C) and 5-fluorouracil (5-FU) treatment allowed exogenous HSC engraftment and hematopoietic reconstitution in WT mice. Our findings provide insight into the molecular basis for the maintenance of HSC quiescence and may lead to improvements in bone marrow transplantation and type I IFN–based therapies for viral infection and cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lengyel, P. Biochemistry of interferons and their actions. Annu. Rev. Biochem. 51, 251–282 (1982).
Stark, G.R., Kerr, I.M., Williams, B.R., Silverman, R.H. & Schreiber, R.D. How cells repond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Pestka, S., Langer, J.A., Zoon, K.C. & Samuel, C.E. Interferons and their actions. Annu. Rev. Biochem. 56, 727–777 (1987).
Biron, C.A. Role of early cytokines, including α and β interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10, 383–390 (1998).
Harada, H. et al. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell 63, 303–312 (1990).
Hida, S. et al. CD8+ T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-α/β signaling. Immunity 13, 643–655 (2000).
Cheshier, S.H., Morrison, S.J., Liao, X. & Weissman, I.L. In vivo proliferation and cell cycle kinetics of long-term self-renewing haematopoietic stem cells. Proc. Natl. Acad. Sci. USA 96, 3120–3125 (1999).
Arai, F. et al. Tie2/angiopoietin-1 signaling regulates haematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004).
Van Zant, G. & Liang, Y. The role of stem cells in aging. Exp. Hematol. 31, 659–672 (2003).
Harrison, D.E. & Astle, C.M. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J. Exp. Med. 156, 1767–1779 (1982).
Hock, H. et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431, 1002–1007 (2004).
Zhang, J. et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 (2006).
Matsuyama, T. et al. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 75, 83–97 (1993).
Mizutani, T. et al. Homeostatic erythropoiesis by the transcription factor IRF2 through attenuation of type I interferon signaling. Exp. Hematol. 36, 255–264 (2008).
Rossi, D.J. et al. Cell intrinsic alterations underlie haematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 102, 9194–9199 (2005).
Kolumam, G.A., Thomas, S., Thompson, L.J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Hüttmann, A., Liu, S.L., Boyd, A.W. & Li, C.L. Functional heterogeneity within rhodamine123lo Hoechst33342lo/sp primitive hemopoietic stem cells revealed by pyronin Y. Exp. Hematol. 29, 1109–1116 (2001).
Yoshihara, H. et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685–697 (2007).
Qian, H. et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1, 671–684 (2007).
Pawliuk, R., Eaves, C. & Humphries, R.K. Evidence of both ontogeny and transplant dose-regulated expansion of hematopoietic stem cells in vivo. Blood 88, 2852–2858 (1996).
Essers, M.A. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Taniguchi, T. & Takaoka, A. A weak signal for strong responses: interferon-α/β revisited. Nat. Rev. Mol. Cell Biol. 2, 378–386 (2001).
Honda, K., Mizutani, T. & Taniguchi, T. Negative regulation of IFN-α/β signaling by IFN regulatory factor 2 for homeostatic development of dendritic cells. Proc. Natl. Acad. Sci. USA 101, 2416–2421 (2004).
Ichikawa, E. et al. Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc. Natl. Acad. Sci. USA 101, 3909–3914 (2004).
Takayanagi, H. et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 416, 744–749 (2002).
Lerner, C. & Harrison, D.E. 5-Fluorouracil spares hematopoietic stem cells responsible for long-term repopulation. Exp. Hematol. 18, 114–118 (1990).
Arai, F. & Suda, T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann. NY Acad. Sci. 1106, 41–53 (2007).
Acknowledgements
We thank Y. Abe and A. Obata-Onai for excellent animal care, K. Yamashita for experimental support, T.W. Mak (Campbell Family Institute for Breast Cancer Research, Toronto) and T. Taniguchi (the University of Tokyo) for the Irf2−/− mice and S. Taki (Shinshu University) for the Irf2−/−Ifnar1−/− mice. This work was supported in part by a Grant-in-Aid for Young Scientists (B) (to T. Sato), a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan (T.O.) and a Grant-in-Aid for the Global Center of Excellence (COE) Program.
Author information
Authors and Affiliations
Contributions
T. Sato conceived of the study, performed experiments, analyzed data and wrote the manuscript. N.O. assisted T. Sato with some of the experiments. H.Y. and F.A. performed Dynamic Array analysis. N.O., F.A., and T. Suda provided discussion and advice on some parts of study. T.O. supervised the overall project and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1040 kb)
Rights and permissions
About this article
Cite this article
Sato, T., Onai, N., Yoshihara, H. et al. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon–dependent exhaustion. Nat Med 15, 696–700 (2009). https://doi.org/10.1038/nm.1973
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1973
This article is cited by
-
Mesenchymal stem cells (MSCs) from the mouse bone marrow show differential expression of interferon regulatory factors IRF-1 and IRF-2
Molecular Biology Reports (2024)
-
Rapid activation of hematopoietic stem cells
Stem Cell Research & Therapy (2023)
-
APEX1 Nuclease and Redox Functions are Both Essential for Adult Mouse Hematopoietic Stem and Progenitor Cells
Stem Cell Reviews and Reports (2023)
-
Myeloid cells promote interferon signaling-associated deterioration of the hematopoietic system
Nature Communications (2022)
-
p53-dependent induction of P2X7 on hematopoietic stem and progenitor cells regulates hematopoietic response to genotoxic stress
Cell Death & Disease (2021)