Abstract
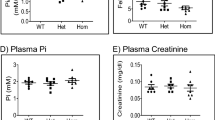
Activation of osteoclasts and their acidification-dependent resorption of bone is thought to maintain proper serum calcium levels. Here we show that osteoclast dysfunction alone does not generally affect calcium homeostasis. Indeed, mice deficient in Src, encoding a tyrosine kinase critical for osteoclast activity, show signs of osteopetrosis, but without hypocalcemia or defects in bone mineralization. Mice deficient in Cckbr, encoding a gastrin receptor that affects acid secretion by parietal cells, have the expected defects in gastric acidification but also secondary hyperparathyroidism and osteoporosis and modest hypocalcemia. These results suggest that alterations in calcium homeostasis can be driven by defects in gastric acidification, especially given that calcium gluconate supplementation fully rescues the phenotype of the Cckbr-mutant mice. Finally, mice deficient in Tcirg1, encoding a subunit of the vacuolar proton pump specifically expressed in both osteoclasts and parietal cells, show hypocalcemia and osteopetrorickets. Although neither Src- nor Cckbr-deficient mice have this latter phenotype, the combined deficiency of both genes results in osteopetrorickets. Thus, we find that osteopetrosis and osteopetrorickets are distinct phenotypes, depending on the site or sites of defective acidification (pages 610–612).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Harada, S. & Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nature 423, 349–355 (2003).
Teitelbaum, S.L. & Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003).
Karaplis, A.C. & Goltzman, D. PTH and PTHrP effects on the skeleton. Rev. Endocr. Metab. Disord. 1, 331–341 (2000).
Kaplan, F.S., August, C.S., Fallon, M.D., Gannon, F. & Haddad, J.G. Osteopetrorickets. The paradox of plenty. Pathophysiology and treatment. Clin. Orthop. Relat. Res. 294, 64–78 (1993).
Taranta, A. et al. Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis. Am. J. Pathol. 162, 57–68 (2003).
Kirubakaran, C., Ranjini, K., Scott, J.X., Basker, M. & Sridhar, G. Osteopetrorickets. J. Trop. Pediatr. 50, 185–186 (2004).
Del Fattore, A., Cappariello, A. & Teti, A. Genetics, pathogenesis and complications of osteopetrosis. Bone 42, 19–29 (2008).
Sly, W.S. et al. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N. Engl. J. Med. 313, 139–145 (1985).
Kornak, U. et al. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104, 205–215 (2001).
Lange, P.F., Wartosch, L., Jentsch, T.J. & Fuhrmann, J.C. ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 440, 220–223 (2006).
Nishi, T. & Forgac, M. The vacuolar H+-ATPases—nature's most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3, 94–103 (2002).
Li, Y.P., Chen, W., Liang, Y., Li, E. & Stashenko, P. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat. Genet. 23, 447–451 (1999).
Scimeca, J.C. et al. The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26, 207–213 (2000).
Marks, S.C. Jr., Seifert, M.F. & Lane, P.W. Osteosclerosis, a recessive skeletal mutation on chromosome 19 in the mouse. J. Hered. 76, 171–176 (1985).
Frattini, A. et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat. Genet. 25, 343–346 (2000).
Kornak, U. et al. Mutations in the a3 subunit of the vacuolar H+-ATPase cause infantile malignant osteopetrosis. Hum. Mol. Genet. 9, 2059–2063 (2000).
Sobacchi, C. et al. The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum. Mol. Genet. 10, 1767–1773 (2001).
Del Fattore, A. et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J. Med. Genet. 43, 315–325 (2006).
Banco, R., Seifert, M.F., Marks, S.C. Jr. & McGuire, J.L. Rickets and osteopetrosis: the osteosclerotic (oc) mouse. Clin. Orthop. Relat. Res. 201, 238–246 (1985).
Li, Y.P., Chen, W. & Stashenko, P. Molecular cloning and characterization of a putative novel human osteoclast-specific 116-kDa vacuolar proton pump subunit. Biochem. Biophys. Res. Commun. 218, 813–821 (1996).
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Langhans, N. et al. Abnormal gastric histology and decreased acid production in cholecystokinin-B/gastrin receptor-deficient mice. Gastroenterology 112, 280–286 (1997).
Gawenis, L.R. et al. Mice with a targeted disruption of the AE2 Cl−/HCO3− exchanger are achlorhydric. J. Biol. Chem. 279, 30531–30539 (2004).
Wilson, C.J. & Velodi, A. Autosomal recessive osteopetrosis: diagnosis, management, and outcome. Arch. Dis. Child. 83, 449–452 (2000).
Driessen, G.J. et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 32, 657–663 (2003).
Frattini, A. et al. Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J. Bone Miner. Res. 18, 1740–1747 (2003).
Kasper, D. et al. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 24, 1079–1091 (2005).
Pangrazio, A. et al. Mutations in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J. Bone Miner. Res. 21, 1098–1105 (2006).
Chalhoub, N. et al. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat. Med. 9, 399–406 (2003).
Kassarjian, Z. & Russell, R.M. Hypochlorhydria: a factor in nutrition. Annu. Rev. Nutr. 9, 271–285 (1989).
Aoki, K. et al. Comparison of prevalence of chronic atrophic gastritis in Japan, China, Tanzania, and the Dominican Republic. Ann. Epidemiol. 15, 598–606 (2005).
Jacobson, B.C. et al. Who is using chronic acid suppression therapy and why? Am. J. Gastroenterol. 98, 51–58 (2003).
Recker, R.R. Calcium absorption and achlorhydria. N. Engl. J. Med. 313, 70–73 (1985).
O'Connell, M.B., Madden, D.M., Murray, A.M., Heaney, R.P. & Kerzner, L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am. J. Med. 118, 778–781 (2005).
Yang, Y.-X., Lewis, J.D., Epstein, S. & Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. J. Am. Med. Assoc. 296, 2947–2953 (2006).
Cummings, S.R. & Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 359, 1761–1767 (2002).
Straub, D.A. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr. Clin. Pract. 22, 286–296 (2007).
Seitz, S. et al. Paget's disease of bone—histologic analysis of 754 patients. J. Bone Miner. Res. 24, 62–69 (2009).
Parfitt, A.M. et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2, 595–610 (1987).
Huebner, A.K. et al. Calcitonin deficiency in mice progressively results in high bone turnover. J. Bone Miner. Res. 21, 1924–1934 (2006).
Hoff, A.O. et al. Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J. Clin. Invest. 110, 1849–1857 (2002).
Schmidt, K. et al. The high mobility group transcription factor Sox8 is a negative regulator of osteoblast differentiation. J. Cell Biol. 168, 899–910 (2005).
Amling, M. et al. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J. Cell Biol. 136, 205–213 (1997).
Simon, R., Mirlacher, M. & Sauter, G. Tissue microarrays. Biotechniques 36, 98–105 (2004).
Takagi, H., Jhappan, C., Sharp, R. & Merlino, G. Hypertrophic gastropathy resembling Ménétrier's disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J. Clin. Invest. 90, 1161–1167 (1992).
Acknowledgements
We thank O. Winter, M. Dietzmann, C. Erdmann, T.O. Klatte, S. Kessler, G. Arndt and S. Conrad for technical assistance. Moreover, we are grateful to A.S. Kopin (Tufts Medical Center) and G.E. Shull (University of Cincinnati) for providing the Cckbr−/− and Slc4a2+/− mice, respectively. This work was supported by grants from the Deutsche Forschungsgemeinschaft to M.A. (AM103/14-1) and M.B. (BL423/4-3), from the Deutsches Zentrum für Luft- und Raumfahrt within the framework of the E-Rare JTC 2007 to M.A., A.S., U.K., A.T. and A.V., from Telethon to A.T. (GGP06119) and by the NOBEL program from Fondazione Cariplo to A.V.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schinke, T., Schilling, A., Baranowsky, A. et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med 15, 674–681 (2009). https://doi.org/10.1038/nm.1963
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1963
This article is cited by
-
The Rogdi knockout mouse is a model for Kohlschütter–Tönz syndrome
Scientific Reports (2024)
-
Osteopetrorickets: two contradictory patterns—one unifying diagnosis
Skeletal Radiology (2024)
-
Effects of more natural housing conditions on the muscular and skeletal characteristics of female C57BL/6J mice
Laboratory Animal Research (2023)
-
Is Helicobacter pylori infection associated with osteoporosis? a systematic review and meta-analysis
Journal of Bone and Mineral Metabolism (2023)
-
Deterioration of apatite orientation in the cholecystokinin B receptor gene (Cckbr)-deficient mouse femurs
Journal of Bone and Mineral Metabolism (2023)