Abstract
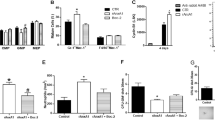
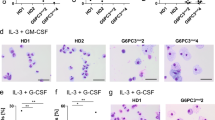
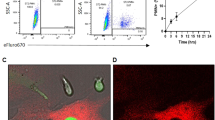
We identified nicotinamide phosphoribosyltransferase (NAMPT), also known as pre-B cell colony enhancing factor (PBEF), as an essential enzyme mediating granulocyte colony-stimulating factor (G-CSF)-triggered granulopoiesis in healthy individuals and in individuals with severe congenital neutropenia. Intracellular NAMPT and NAD+ amounts in myeloid cells, as well as plasma NAMPT and NAD+ levels, were increased by G-CSF treatment of both healthy volunteers and individuals with congenital neutropenia. NAMPT administered both extracellularly and intracellularly induced granulocytic differentiation of CD34+ hematopoietic progenitor cells and of the promyelocytic leukemia cell line HL-60. Treatment of healthy individuals with high doses of vitamin B3 (nicotinamide), a substrate of NAMPT, induced neutrophilic granulocyte differentiation. The molecular events triggered by NAMPT include NAD+-dependent sirtuin-1 activation, subsequent induction of CCAAT/enhancer binding protein-α and CCAAT/enhancer binding protein-β, and, ultimately, upregulation of G-CSF synthesis and G-CSF receptor expression. G-CSF, in turn, further increases NAMPT levels. These results reveal a decisive role of the NAD+ metabolic pathway in G-CSF-triggered myelopoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Welte, K. et al. Filgrastim (r-metHuG-CSF): the first 10 years. Blood 88, 1907–1929 (1996).
Welte, K., Zeidler, C. & Dale, D.C. Severe congenital neutropenia. Semin. Hematol. 43, 189–195 (2006).
Skokowa, J. et al. Severe congenital neutropenia: inheritance and pathophysiology. Curr. Opin. Hematol. 14, 22–28 (2007).
Kyas, U., Pietsch, T. & Welte, K. Expression of receptors for granulocyte colony-stimulating factor on neutrophils from patients with severe congenital neutropenia and cyclic neutropenia. Blood 79, 1144–1147 (1992).
Skokowa, J. et al. LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat. Med. 12, 1191–1197 (2006).
Hirai, H. et al. C/EBPbeta is required for 'emergency' granulopoiesis. Nat. Immunol. 7, 732–739 (2006).
Rongvaux, A. et al. Pre-B cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 32, 3225–3234 (2002).
Belenky, P., Bogan, K.L. & Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 (2007).
Revollo, J.R., Grimm, A.A. & Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 (2004).
Ziegler, M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur. J. Biochem. 267, 1550–1564 (2000).
Vaziri, H. et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001).
Giannakou, M.E. & Partridge, L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 14, 408–412 (2004).
De Flora, A. et al. Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. NY Acad. Sci. 1028, 176–191 (2004).
Samal, B. et al. Cloning and characterization of the cDNA encoding a novel human pre-B cell colony-enhancing factor. Mol. Cell. Biol. 14, 1431–1437 (1994).
Jia, S.H. et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Invest. 113, 1318–1327 (2004).
Nowell, M.A. et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 54, 2084–2095 (2006).
Kendal, C.E. & Bryant-Greenwood, G.D. Pre-B cell colony-enhancing factor (PBEF/Visfatin) gene expression is modulated by NF-κB and AP-1 in human amniotic epithelial cells. Placenta 28, 305–314 (2007).
Iqbal, J. & Zaidi, M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem. Biophys. Res. Commun. 342, 1312–1318 (2006).
van der Veer, E. et al. Pre-B cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ. Res. 97, 25–34 (2005).
Sasaki, Y., Araki, T. & Milbrandt, J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J. Neurosci. 26, 8484–8491 (2006).
Revollo, J.R. et al. NAMPT/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 6, 363–375 (2007).
Hasmann, M. & Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 63, 7436–7442 (2003).
Yang, T. & Sauve, A.A. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 8, E632–E643 (2006).
Tanaka, T. & Nabeshima, Y. NAMPT/Visfatin: a new player in beta cell physiology and in metabolic diseases? Cell Metab. 6, 341–343 (2007).
Khan, J.A., Tao, X. & Tong, L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 13, 582–588 (2006).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Blander, G. & Guarente, L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73, 417–435 (2004).
Sauve, A.A. et al. The biochemistry of sirtuins. Annu. Rev. Biochem. 75, 435–465 (2006).
Kaneko, S. et al. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26, 9794–9804 (2006).
Araki, T., Sasaki, Y. & Milbrandt, J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 (2004).
Prozorovski, T. et al. SIRT1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 10, 385–394 (2008).
Libert, S., Cohen, D. & Guarente, L. Neurogenesis directed by SIRT1. Nat. Cell Biol. 10, 373–374 (2008).
Han, M.K. et al. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2, 241–251 (2008).
Qiao, L. & Shao, J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein α transcriptional complex. J. Biol. Chem. 281, 39915–39924 (2006).
Xu, M. et al. STAT5-induced Id-1 transcription involves recruitment of HDAC1 and deacetylation of C/EBPβ. EMBO J. 22, 893–904 (2003).
Smith, L.T. et al. PU.1 (Spi-1) and C/EBP α regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood 88, 1234–1247 (1996).
Lenny, N., Westendorf, J.J. & Hiebert, S.W. Transcriptional regulation during myelopoiesis. Mol. Biol. Rep. 24, 157–168 (1997).
Dale, D.C. et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood 96, 2317–2322 (2000).
Klein, C. et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat. Genet. 39, 86–92 (2007).
Knip, M. et al. Safety of high-dose nicotinamide: a review. Diabetologia 43, 1337–1345 (2000).
Sauve, A.A. NAD+ and vitamin B3: from metabolism to therapies. J. Pharmacol. Exp. Ther. 324, 883–893 (2008).
Kasper, B., Welte, K. & Hadam, M.R. In Leukocyte Typing IV (eds. Kishimoto, T. et al.) 1072–1074 (Garland Publishing, New York, 1997).
Acknowledgements
We thank M. Uenalan for helpful discussion, M. Morgan for critically reading the manuscript and M. Ballmaier and C. Reimer for assistance in cell sorting. We also thank C. Zeidler and the physicians within the Severe Chronic Neutropenia International Registry for providing subject material (bone marrow cells, peripheral blood neutrophils and plasma). Special thanks to study subjects and colleagues, especially M. Schatz, for their great cooperation. This work was supported by Elternverein Krebskranker Kinder Hannover e.V., Madeleine-Schickedanz-Kinderkrebsstiftung, Deutsche José Carreras Leukämia Stiftung e.V., REBIRTH Excellence Cluster (A.S.), Else Kröner Foundation (A.S.), an American Federation for Aging Research senior postdoctoral fellowship (F.W.) and the US Department of Agriculture and National Institutes of Health (Q.T.).
Author information
Authors and Affiliations
Contributions
K.W. and J.S. made initial observations, designed the main experiments, analyzed the data, supervised experimentation and wrote the manuscript; J.S. and D.L. performed the main experiments; B.K.T. performed western blotting and immunoprecipitation experiments; A.M.B. cloned and prepared plasmids for transfection experiments; K.G. introduced mutations into G-CSF and G-CSFR reporter gene constructs; L.H. cloned G-CSFR shRNA; F.W. and Q.T. provided plasmids for SIRT1, C/EBP-α and C/EBP-β, performed immunoprecipitation experiments in 293T cells and discussed the manuscript; A.S. designed NAMPT lv constructs and produced viral supernatants; G.C., M.G. and M.S. discussed the project and manuscript; G.M. and M.G. provided FK866 and helped to measure NAD+; K.W. coordinated the project and wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–9 and Supplementary Methods (PDF 1735 kb)
Rights and permissions
About this article
Cite this article
Skokowa, J., Lan, D., Thakur, B. et al. NAMPT is essential for the G-CSF–induced myeloid differentiation via a NAD+–sirtuin-1–dependent pathway. Nat Med 15, 151–158 (2009). https://doi.org/10.1038/nm.1913
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1913
This article is cited by
-
NAD salvage pathway machinery expression in normal and glaucomatous retina and optic nerve
Acta Neuropathologica Communications (2023)
-
Intertwining roles of circadian and metabolic regulation of the innate immune response
Seminars in Immunopathology (2022)
-
NAMPT/SIRT2-mediated inhibition of the p53-p21 signaling pathway is indispensable for maintenance and hematopoietic differentiation of human iPS cells
Stem Cell Research & Therapy (2021)
-
MLKL promotes cellular differentiation in myeloid leukemia by facilitating the release of G-CSF
Cell Death & Differentiation (2021)
-
Implications of metabolism-driven myeloid dysfunctions in cancer therapy
Cellular & Molecular Immunology (2021)