Abstract
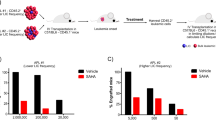
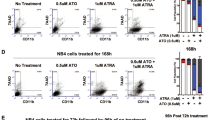
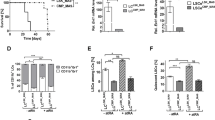
Retinoic acid and arsenic trioxide target the protein stability and transcriptional repression activity of the fusion oncoprotein PML-RARA, resulting in regression of acute promyelocytic leukemia (APL). Phenotypically, retinoic acid induces differentiation of APL cells. Here we show that retinoic acid also triggers growth arrest of leukemia-initiating cells (LICs) ex vivo and their clearance in PML-RARA mouse APL in vivo. Retinoic acid treatment of mouse APLs expressing the fusion protein PLZF-RARA triggers full differentiation, but not LIC loss or disease remission, establishing that differentiation and LIC loss can be uncoupled. Although retinoic acid and arsenic synergize to clear LICs through cooperative PML-RARA degradation, this combination does not enhance differentiation. A cyclic AMP (cAMP)-dependent phosphorylation site in PML-RARA is crucial for retinoic acid–induced PML-RARA degradation and LIC clearance. Moreover, activation of cAMP signaling enhances LIC loss by retinoic acid, identifying cAMP as another potential APL therapy. Thus, whereas transcriptional activation of PML-RARA is likely to control differentiation, its catabolism triggers LIC eradication and long-term remission of mouse APL. Therapy-triggered degradation of oncoproteins could be a general strategy to eradicate cancer stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kamashev, D.E., Vitoux, D. & de Thé, H. PML/RARA-RXR oligomers mediate retinoid- and rexinoid- /cAMP in APL cell differentiation. J. Exp. Med. 199, 1163–1174 (2004).
van Wageningen, S. et al. Gene transactivation without direct DNA binding defines a novel gain-of-function for PML-RAR{alpha}. Blood 111, 1634–1643 (2008).
Melnick, A. & Licht, J.D. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 93, 3167–3215 (1999).
Di Croce, L. et al. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science 295, 1079–1082 (2002).
Zhu, J. et al. A sumoylation site in PML/RARA is essential for leukemic transformation. Cancer Cell 7, 143–153 (2005).
Zhou, J. et al. Dimerization-induced corepressor binding and relaxed DNA-binding specificity are critical for PML/RARA-induced immortalization. Proc. Natl. Acad. Sci. USA 103, 9238–9243 (2006).
Villa, R. et al. Role of the polycomb repressive complex 2 in acute promyelocytic leukemia. Cancer Cell 11, 513–525 (2007).
Zhu, J. et al. RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell 12, 23–35 (2007).
Zeisig, B.B. et al. Recruitment of RXR by homotetrameric RARalpha fusion proteins is essential for transformation. Cancer Cell 12, 36–51 (2007).
Quignon, F., Chen, Z. & de Thé, H. Retinoic acid and arsenic: towards oncogene targeted treatments of acute promyelocytic leukaemia. Biochim. Biophys. Acta 1333, M53–M61 (1997).
Zhu, J., Lallemand-Breitenbach, V. & de The, H. Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission. Oncogene 20, 7257–7265 (2001).
Wang, Z.Y. & Chen, Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111, 2505–2515 (2008).
Muindi, J. et al. Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid “resistance” in patients with acute promyelocytic leukemia. Blood 79, 299–303 (1992).
Nervi, C. et al. Caspases mediate retinoic acid-induced degradation of the acute promyelocytic leukemia PML/RARalpha fusion protein. Blood 92, 2244–2251 (1998).
Zhu, J. et al. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RAR alpha) and oncogenic RAR alpha fusion proteins. Proc. Natl. Acad. Sci. USA 96, 14807–14812 (1999).
Lane, A.A. & Ley, T.J. Neutrophil elastase cleaves PML-RARalpha and is important for the development of acute promyelocytic leukemia in mice. Cell 115, 305–318 (2003).
vom Baur, E. et al. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 15, 110–124 (1996).
Lallemand-Breitenbach, V. et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As(2)O(3)-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 193, 1361–1372 (2001).
Mann, K.K. et al. Arsenic trioxide inhibits nuclear receptor function via SEK1/JNK-mediated RXRalpha phosphorylation. J. Clin. Invest. 115, 2924–2933 (2005).
Zhu, J. et al. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 94, 3978–3983 (1997).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10, 547–555 (2008).
Hayakawa, F. & Privalsky, M.L. Phosphorylation of PML by mitogen-activated protein kinases plays a key role in arsenic trioxide-mediated apoptosis. Cancer Cell 5, 389–401 (2004).
Lallemand-Breitenbach, V. et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 189, 1043–1052 (1999).
Rego, E.M., He, L.Z., Warrell, R.P., Jr., Wang, Z.G. & Pandolfi, P.P. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc. Natl. Acad. Sci. USA 97, 10173–10178 (2000).
Westervelt, P. et al. Adaptive immunity cooperates with liposomal all-trans-retinoic acid (ATRA) to facilitate long-term molecular remissions in mice with acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 99, 9468–9473 (2002).
Lallemand-Breitenbach, V., Zhu, J., Kogan, S., Chen, Z. & de The, H. Opinion: how patients have benefited from mouse models of acute promyelocytic leukaemia. Nat. Rev. Cancer 5, 821–827 (2005).
Shen, Z.X. et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 101, 5328–5335 (2004).
Estey, E. et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107, 3469–3473 (2006).
Shao, W. et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RARalpha protein in acute promyelocytic leukemia cells. J. Natl. Cancer Inst. 90, 124–133 (1998).
Ruchaud, S. et al. Two distinctly regulated events, priming and triggering, during retinoid-induced maturation and resistance of NB4 promyelocytic leukemia cell line. Proc. Natl. Acad. Sci. USA 91, 8428–8432 (1994).
Guillemin, M.C. et al. In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia. J. Exp. Med. 196, 1373–1380 (2002).
Altucci, L. et al. Rexinoid-triggered differentiation and tumours selective apoptosis of AML by protein kinase-A-mediated de-subordination of RXR. Cancer Res. 65, 8754–8765 (2005).
Gaillard, E. et al. Phosphorylation by PKA potentiates retinoic acid receptor alpha activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc. Natl. Acad. Sci. USA 103, 9548–9553 (2006).
Wang, J.C. & Dick, J.E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005).
Zheng, X. et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica 92, 323–331 (2007).
He, L.Z. et al. Two critical hits for promyelocytic leukemia. Mol. Cell 6, 1131–1141 (2000).
He, L.-Z. et al. Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat. Genet. 18, 126–135 (1998).
Parrella, E. et al. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the cAMP and the retinoic acid signaling pathways. J. Biol. Chem. 279, 42026–42040 (2004).
Zhu, J., Chen, Z., Lallemand-Breitenbach, V. & de Thé, H. How acute promyelocytic leukemia revived arsenic. Nat. Rev. Cancer 2, 705–713 (2002).
Turhan, A.G. et al. Highly purified primitive hematopoietic stem cells are PML-RARA negative and generate nonclonal progenitors in acute promyelocytic leukemia. Blood 85, 2154–2161 (1995).
Zheng, P.Z. et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 102, 7653–7658 (2005).
Lin, D.Y. et al. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell 24, 341–354 (2006).
Ito, K. et al. PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453, 1072–1078 (2008).
Tsimberidou, A.M. et al. Single-agent liposomal all-trans retinoic acid can cure some patients with untreated acute promyelocytic leukemia: an update of The University of Texas M. D. Anderson Cancer Center Series. Leuk. Lymphoma 47, 1062–1068 (2006).
Chen, G.Q. et al. Pharmacokinetics and efficacy of low-dose all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Leukemia 10, 825–828 (1996).
Koken, M.H.M. et al. Retinoic acid, but not arsenic trioxide, degrades the PLZF/RARalpha fusion protein, without inducing terminal differentiation or apoptosis, in a RA-therapy resistant t(11;17)(q23;q21) APL patient. Oncogene 18, 1113–1118 (1999).
Costoya, J.A. et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659 (2004).
Khetchoumian, K. et al. Loss of Trim24 (Tif1alpha) gene function confers oncogenic activity to retinoic acid receptor alpha. Nat. Genet. 39, 1500–1506 (2007).
Purton, L.E. et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 203, 1283–1293 (2006).
Matthay, K.K. et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N. Engl. J. Med. 341, 1165–1173 (1999).
Acknowledgements
R.N. was supported by the Lady Tata Foundation (London). This work was supported by the ARECA, EPITRON (an integrated project funded by the European Union under the sixth framework program (LSHC-CT-2005-518417)) and INCa/Canceropole programs. We thank J. Godet and the Comité des Yvelines de la Ligue contre le Cancer for their continuous support of this project; M. Pla and the animal housing facility; C. Leboeuf, L. Legrès and A. Janin for facilitation of the pathological analysis of the mice; M. Kawatika, M. Giovanini and F. Riaucoux for the derivation of MRP8-PML-RARAS873A transgenic mice; P. Chambon for the antibody to RARA; S. Kogan for the APLs and the MSCV-luciferase vector; B. Arnulf for bortezomib; H. Tenor (Altana/Nycomed) for piclamilast; O. Hermine and F. Valensi for help with the t(11;17) human data; the Treilles and IPSEN foundations for providing the setting where this work was first presented and developed; and A. Saib, J.C. Gluckman and F. Sigaux for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
CNRS UMR7151 has had contacts with ALTANA (now Nycomed), a company that has interest in phosphodiesterase inhibitors (PDEIs). ALTANA provided the authors with piclamilast, a reagent that was use in these studies but does not belong to ALTANA. No financial support was provided toward experiments reported in this study. On the basis of the results reported in this study, Paris 7 University has contracted the testing of another PDEI on the APL animal model used in the study, with financial support from Nycomed to CNRS UMR7151. Paris 7 University has filed a patent to the European patent office for the eradication of LIC through PML-RARA degradation.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1—5 and Supplementary Table 1 (PDF 1983 kb)
Rights and permissions
About this article
Cite this article
Nasr, R., Guillemin, MC., Ferhi, O. et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med 14, 1333–1342 (2008). https://doi.org/10.1038/nm.1891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1891
This article is cited by
-
Co-targeting leukemia-initiating cells and leukemia bulk leads to disease eradication
Leukemia (2022)
-
A novel network pharmacology approach for leukaemia differentiation therapy using Mogrify®
Oncogene (2022)
-
(–)-Epigallocatechin-3-gallate induces apoptosis and differentiation in leukaemia by targeting reactive oxygen species and PIN1
Scientific Reports (2021)
-
A novel fusion protein TBLR1-RARα acts as an oncogene to induce murine promyelocytic leukemia: identification and treatment strategies
Cell Death & Disease (2021)
-
Targeting DUBs to degrade oncogenic proteins
British Journal of Cancer (2020)