Abstract
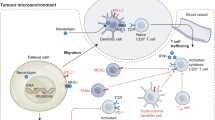
Genetic and epigenetic plasticity allows tumors to evade single-targeted treatments. Here we direct Bcl2-specific short interfering RNA (siRNA) with 5′-triphosphate ends (3p-siRNA) against melanoma. Recognition of 5′-triphosphate by the cytosolic antiviral helicase retinoic acid–induced protein I (Rig-I, encoded by Ddx58) activated innate immune cells such as dendritic cells and directly induced expression of interferons (IFNs) and apoptosis in tumor cells. These Rig-I–mediated activities synergized with siRNA-mediated Bcl2 silencing to provoke massive apoptosis of tumor cells in lung metastases in vivo. The therapeutic activity required natural killer cells and IFN, as well as silencing of Bcl2, as evidenced by rescue with a mutated Bcl2 target, by site-specific cleavage of Bcl2 messenger RNA in lung metastases and downregulation of Bcl-2 protein in tumor cells in vivo. Together, 3p-siRNA represents a single molecule–based approach in which Rig-I activation on both the immune- and tumor cell level corrects immune ignorance and in which gene silencing corrects key molecular events that govern tumor cell survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanahan, D. & Weinberg, R.A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Bui, J.D. & Schreiber, R.D. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr. Opin. Immunol. 19, 203–208 (2007).
Rubin, B.P., Heinrich, M.C. & Corless, C.L. Gastrointestinal stromal tumour. Lancet 369, 1731–1741 (2007).
Curiel, T.J. Tregs and rethinking cancer immunotherapy. J. Clin. Invest. 117, 1167–1174 (2007).
Uno, T. et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat. Med. 12, 693–698 (2006).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Schlee, M., Hornung, V. & Hartmann, G. siRNA and isRNA: two edges of one sword. Mol. Ther. 14, 463–470 (2006).
Pei, Y. & Tuschl, T. On the art of identifying effective and specific siRNAs. Nat. Methods 3, 670–676 (2006).
de Fougerolles, A., Vornlocher, H.P., Maraganore, J. & Lieberman, J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 6, 443–453 (2007).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA–induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Hornung, V. et al. 5′-triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Pichlmair, A. et al. RIG-I–mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Yoneyama, M. & Fujita, T. Function of RIG-I–like receptors in antiviral innate immunity. J. Biol. Chem. 282, 15315–15318 (2007).
Kim, D.H. et al. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat. Biotechnol. 22, 321–325 (2004).
Miller, A.J. & Mihm, M.C. Jr. Melanoma. N. Engl. J. Med. 355, 51–65 (2006).
Danial, N.N. & Korsmeyer, S.J. Cell death: critical control points. Cell 116, 205–219 (2004).
McGill, G.G. et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109, 707–718 (2002).
Hornung, V. et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11, 263–270 (2005).
Judge, A.D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 23, 457–462 (2005).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 (2006).
Kato, H. et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23, 19–28 (2005).
Melchjorsen, J. et al. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J. Virol. 79, 12944–12951 (2005).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I– and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Tormo, D. et al. Therapeutic efficacy of antigen-specific vaccination and Toll-like receptor stimulation against established transplanted and autochthonous melanoma in mice. Cancer Res. 66, 5427–5435 (2006).
Stetson, D.B. & Medzhitov, R. Antiviral defense: interferons and beyond. J. Exp. Med. 203, 1837–1841 (2006).
Diebold, S.S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Muller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Kamphuis, E., Junt, T., Waibler, Z., Forster, R. & Kalinke, U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108, 3253–3261 (2006).
Mocikat, R. et al. Natural killer cells activated by MHC class Ilow targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 19, 561–569 (2003).
Acknowledgements
This study was supported by grant Biofuture 0311896 of the Bundesministerium für Bildung und Forschung and by grants SFB 704, SFB 670, KFO115 and KFO177 of the Deutsche Forschungsgemeinschaft to G. Hartmann; by grants Tu90-6/1 of the Deutsche Forschungsgemeinschaft and 10741 of the Deutsche Krebshilfe to T.T.; by grant 107805 of the Deutsche Krebshilfe to R.B.; by Graduiertenkolleg 1202 to C.M., M.B. and T.S.; by LMUexcellent (CIPSM 114, research professorship), and by SFB-TR 36 and En 169/7-2 of the Deutsche Forschungsgemeinschaft to S.E. and C. Bourquin. This work is part of the theses of C.M. and M.B. at the University of Munich and of M.R. and J.L. at the University of Bonn. We thank A. Dann for excellent technical assistance, T. Maniatis (Harvard University) and J. Chen (University of Texas Southwestern Medical Center) for providing IFN-β–Luc reporter plasmids and wild-type pPME-myc NS3-4A (NS3-4A), respectively, T. Fujita Institute for Virus Research, Kyoto University for providing Rig-I and the empty control vector and C. Borner (Institute of Molecular Medicine and Cell Research, University of Freiburg) for providing wild-type mouse Bcl-2 (mBcl-2/pcDNA).
Author information
Authors and Affiliations
Contributions
H.P., R.B., T.T. and G. Hartmann designed the research; H.P., C.M., R.B., D.T., S.S.M., M.R., S.K., E.G., J.L., J.H., A.S., M.B., T.S., D.A., M.N., M.P., C. Berking and T.T. performed experiments; H.P., R.B., S.E., C. Bourquin, G. Häcker, C. Berking, R.M., V.H., S.R., T.T. and G. Hartmann conducted the data analyses; E.K., U.K., D.B., J.R., H.K. and S.A. provided genetically deficient mice and key research tools; H.P., C.M., R.B. and T.T. prepared the figures; and H.P., T.T. and G. Hartmann wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.S.M. and R.M., as employees of Alnylam Pharmaceuticals, have options to purchase shares of Alnylam stock, and R.M. owns Alnylam stock.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3, Supplementary Figs. 1–10 and Supplementary Methods (PDF 2137 kb)
Rights and permissions
About this article
Cite this article
Poeck, H., Besch, R., Maihoefer, C. et al. 5′-triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med 14, 1256–1263 (2008). https://doi.org/10.1038/nm.1887
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1887
This article is cited by
-
Exploiting RIG-I-like receptor pathway for cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Local immunotherapy with the RNA-based immune stimulator CV8102 induces substantial anti-tumor responses and enhances checkpoint inhibitor activity
Cancer Immunology, Immunotherapy (2023)
-
High RIG-I and EFTUD2 expression predicts poor survival in endometrial cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
The RIG-I agonist M8 triggers cell death and natural killer cell activation in human papillomavirus-associated cancer and potentiates cisplatin cytotoxicity
Cancer Immunology, Immunotherapy (2023)
-
Context-dependent functions of pattern recognition receptors in cancer
Nature Reviews Cancer (2022)