Abstract
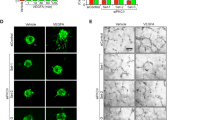
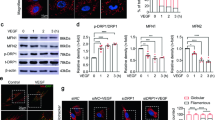
Vascularization is essential for tissue development and in restoration of tissue integrity after an ischemic injury. In studies of vascularization, the focus has largely been placed on vascular endothelial growth factor (VEGF), yet other factors may also orchestrate this process. Here we show that succinate accumulates in the hypoxic retina of rodents and, via its cognate receptor G protein–coupled receptor-91 (GPR91), is a potent mediator of vessel growth in the settings of both normal retinal development and proliferative ischemic retinopathy. The effects of GPR91 are mediated by retinal ganglion neurons (RGCs), which, in response to increased succinate levels, regulate the production of numerous angiogenic factors including VEGF. Accordingly, succinate did not have proangiogenic effects in RGC-deficient rats. Our observations show a pathway of metabolite signaling where succinate, acting through GPR91, governs retinal angiogenesis and show the propensity of RGCs to act as sensors of ischemic stress. These findings provide a new therapeutic target for modulating revascularization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Adair, T.H., Gay, W.J. & Montani, J.P. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am. J. Physiol. 259, R393–R404 (1990).
Aiello, L.P. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res. 29, 354–362 (1997).
Enge, M. et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 21, 4307–4316 (2002).
Smith, L.E. et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science 276, 1706–1709 (1997).
Watanabe, D. et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N. Engl. J. Med. 353, 782–792 (2005).
D'Amore, P.A. Mechanisms of retinal and choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 35, 3974–3979 (1994).
Cai, W., Rook, S.L., Jiang, Z.Y., Takahara, N. & Aiello, L.P. Mechanisms of hepatocyte growth factor–induced retinal endothelial cell migration and growth. Invest. Ophthalmol. Vis. Sci. 41, 1885–1893 (2000).
Folbergrova, J., Ljunggren, B., Norberg, K. & Siesjo, B.K. Influence of complete ischemia on glycolytic metabolites, citric acid cycle intermediates, and associated amino acids in the rat cerebral cortex. Brain Res. 80, 265–279 (1974).
Hoyer, S. & Krier, C. Ischemia and aging brain. Studies on glucose and energy metabolism in rat cerebral cortex. Neurobiol. Aging 7, 23–29 (1986).
Gutman, M., Bonomi, F., Pagani, S., Cerletti, P. & Kroneck, P. Modulation of the flavin redox potential as mode of regulation of succinate dehydrogenase activity. Biochim. Biophys. Acta 591, 400–408 (1980).
Meixner-Monori, B., Kubicek, C.P., Habison, A., Kubicek-Pranz, E.M. & Rohr, M. Presence and regulation of the α-ketoglutarate dehydrogenase multienzyme complex in the filamentous fungus Aspergillus niger. J. Bacteriol. 161, 265–271 (1985).
Mailloux, R.J. et al. The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLoS ONE 2, e690 (2007).
Burns, P.A. & Wilson, D.J. Angiogenesis mediated by metabolites is dependent on vascular endothelial growth factor (VEGF). Angiogenesis 6, 73–77 (2003).
Lee, M.S. et al. Angiogenic activity of pyruvic acid in in vivo and in vitro angiogenesis models. Cancer Res. 61, 3290–3293 (2001).
Murray, B. & Wilson, D.J. A study of metabolites as intermediate effectors in angiogenesis. Angiogenesis 4, 71–77 (2001).
Neuman, R.E. & Mc, C.T. Growth-promoting properties of pyruvate oxal-acetate, and α-ketoglutarate for isolated Walker carcinosarcoma 256 cells. Proc. Soc. Exp. Biol. Med. 98, 303–306 (1958).
He, W. et al. Citric acid cycle intermediates as ligands for orphan G protein–coupled receptors. Nature 429, 188–193 (2004).
Sennlaub, F. et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation 108, 198–204 (2003).
Kushnir, M.M., Komaromy-Hiller, G., Shushan, B., Urry, F.M. & Roberts, W.L. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin. Chem. 47, 1993–2002 (2001).
van Adel, B.A., Kostic, C., Deglon, N., Ball, A.K. & Arsenijevic, Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum. Gene Ther. 14, 103–115 (2003).
Wittenberger, T. et al. GPR99, a new G protein–coupled receptor with homology to a new subgroup of nucleotide receptors. BMC Genomics 3, 17 (2002).
Ogunshola, O.O. et al. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res. Dev. Brain. Res. 119, 139–153 (2000).
Marti, H.H. & Risau, W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc. Natl. Acad. Sci. USA 95, 15809–15814 (1998).
Stowe, A.M. et al. VEGF protein associates to neurons in remote regions following cortical infarct. J. Cereb. Blood Flow Metab. 27, 76–85 (2007).
Calza, L., Giardino, L., Giuliani, A., Aloe, L. & Levi-Montalcini, R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc. Natl. Acad. Sci. USA 98, 4160–4165 (2001).
Iriyama, A., Chen, Y.N., Tamaki, Y. & Yanagi, Y. Effect of anti-VEGF antibody on retinal ganglion cells in rats. Br. J. Ophthalmol. 91, 1230–1233 (2007).
Zhu, T. et al. Proangiogenic effects of protease-activated receptor 2 are tumor necrosis factor-α and consecutively Tie2 dependent. Arterioscler. Thromb. Vasc. Biol. 26, 744–750 (2006).
Eklund, L. & Olsen, B.R. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 312, 630–641 (2006).
Koivunen, P. et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 282, 4524–4532 (2007).
Selak, M.A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005).
McColm, J.R., Geisen, P. & Hartnett, M.E. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol. Vis. 10, 512–520 (2004).
Ikeda, M., Hosoda, Y., Hirose, S., Okada, Y. & Ikeda, E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR and neuropilin-1 in synovial tissues of rheumatoid arthritis. J. Pathol. 191, 426–433 (2000).
Usui, T. et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest. Ophthalmol. Vis. Sci. 45, 368–374 (2004).
Sapieha, P.S., Peltier, M., Rendahl, K.G., Manning, W.C. & Di Polo, A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol. Cell. Neurosci. 24, 656–672 (2003).
Berkelaar, M., Clarke, D.B., Wang, Y.C., Bray, G.M. & Aguayo, A.J. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. 14, 4368–4374 (1994).
Huxlin, K.R., Dreher, Z., Schulz, M. & Dreher, B. Glial reactivity in the retina of adult rats. Glia 15, 105–118 (1995).
Mu, X. et al. Ganglion cells are required for normal progenitor cell proliferation but not cell-fate determination or patterning in the developing mouse retina. Curr. Biol. 15, 525–530 (2005).
Higgins, R.D. et al. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr. Eye Res. 18, 20–27 (1999).
Gariano, R.F. & Gardner, T.W. Retinal angiogenesis in development and disease. Nature 438, 960–966 (2005).
Arjamaa, O. & Nikinmaa, M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp. Eye Res. 83, 473–483 (2006).
Shweiki, D., Itin, A., Soffer, D. & Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843–845 (1992).
Johnson, R.N. & Hansford, R.G. The control of tricarboxylate-cycle oxidations in blowfly flight muscle. The steady-state concentrations of citrate, isocitrate 2-oxoglutarate and malate in flight muscle and isolated mitochondria. Biochem. J. 146, 527–535 (1975).
Hems, D.A. & Brosnan, J.T. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem. J. 120, 105–111 (1970).
Pierce, E.A., Avery, R.L., Foley, E.D., Aiello, L.P. & Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 92, 905–909 (1995).
Smith, L.E. et al. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 35, 101–111 (1994).
Matthews, P.M., Nagy, Z., Brown, G.K., Land, J. & Squier, M.V. Isolated capillary proliferation in Leigh's syndrome. Clin. Neuropathol. 13, 139–141 (1994).
Piao, Y.S., Tang, G.C., Yang, H. & Lu, D.H. Clinico-neuropathological study of a Chinese case of familial adult Leigh syndrome. Neuropathology 26, 218–221 (2006).
Francois, J. & Neetens, A. Comparative anatomy of the vascular supply of the eye in vertebrates. in In The Eye Vol. 5, (ed. H. Davson & L.T. Graham) 1–70 (Academic Press, New York, 1974).
Johnson, G.L. Ophthalmoscopic studies on the eyes of mammals. Phil. Trans. R. Soc. Lond. B2, 1–82 (1968).
Michaelson, I.C. Retinal Circulation in Man and Mammals. (ed. Thomas, C.C.) Ch. 1–9 (Charles C. Thomas, Springfield, Illinois, 1954).
Braun, R.D., Linsenmeier, R.A. & Goldstick, T.K. Oxygen consumption in the inner and outer retina of the cat. Invest. Ophthalmol. Vis. Sci. 36, 542–554 (1995).
Fruttiger, M. et al. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron 17, 1117–1131 (1996).
Hughes, S., Yang, H. & Chan-Ling, T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest. Ophthalmol. Vis. Sci. 41, 1217–1228 (2000).
Dreher, B. & Robinson, S.R. Development of the retinofugal pathway in birds and mammals: evidence for a common 'timetable'. Brain Behav. Evol. 31, 369–390 (1988).
Cringle, S.J., Yu, P.K., Su, E.N. & Yu, D.Y. Oxygen distribution and consumption in the developing rat retina. Invest. Ophthalmol. Vis. Sci. 47, 4072–4076 (2006).
Arany, Z. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451, 1008–1012 (2008).
Frassetto, L.J. et al. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest. Ophthalmol. Vis. Sci. 47, 427–438 (2006).
Dull, T. et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72, 8463–8471 (1998).
Penn, J.S., Henry, M.M. & Tolman, B.L. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr. Res. 36, 724–731 (1994).
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research, the March of Dimes Birth Defects Foundation, the Heart and Stroke Foundation of Québec and the Fonds de la Recherche en Santé du Québec. P.S. and M.S. hold a Research Fellowship Award and a studentship from the Heart and Stroke Foundation of Canada, respectively. K.Z. is a recipient of The Foundation Fighting Blindness Postdoctoral Fellowship Award. F.S. and S.C. are recipients of a fellowship and scientist awards, respectively, from the Canadian Institutes of Health Research. S.C. also holds a Canada Research Chair (perinatology). Generation of brn3bZ-dta/+;six3-cre mice and J.-H.C.'s salary were supported by a US National Eye Institute grant EY011930 to W.H.K. and by the Robert A. Welch Foundation (G-0010), respectively. We wish to thank N. Agarwal, from the University of North Texas Health Science Center at Fort Worth, for his kind donation of the RGC-5 cell line. We also wish to thank H. Fernandez and S. Leclerc for valuable technical assistance.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1-4, Supplementary Table 1 and Supplementary Methods (PDF 1751 kb)
Supplementary Video 1
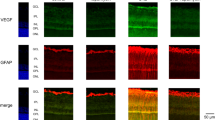
Three-dimensional confocal representation of a 30-μm–thick radial section of the retina. GPR91-positive cells are labeled in green, whereas lectin-stained blood vessels are in red. A lack of overlap indicates that GPR91 is not expressed in retinal blood vessels. (MOV 1060 kb)
Rights and permissions
About this article
Cite this article
Sapieha, P., Sirinyan, M., Hamel, D. et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med 14, 1067–1076 (2008). https://doi.org/10.1038/nm.1873
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1873
This article is cited by
-
GPCRs involved in metabolic diseases: pharmacotherapeutic development updates
Acta Pharmacologica Sinica (2024)
-
Type 2 diabetes and succinate: unmasking an age-old molecule
Diabetologia (2024)
-
Evaluating fine changes in visual function of diabetic eyes using spatial-sweep steady-state pattern electroretinography
Scientific Reports (2023)
-
Neuronal Bmal1 regulates retinal angiogenesis and neovascularization in mice
Communications Biology (2022)
-
Neurovascular abnormalities in retinopathy of prematurity and emerging therapies
Journal of Molecular Medicine (2022)