Abstract
Dendritic cells (DCs) can present extracellularly derived antigens in the context of major histocompatibility complex (MHC) class I molecules, a process called cross-presentation. Although recognized to be important for priming of T cell responses to many viral, bacterial and tumor antigens, the mechanistic details of this alternative antigen-presentation pathway are poorly understood. We demonstrate here the existence of an endolysosomal compartment in DCs where exogenously derived peptides can be acquired for presentation to T cells, and show that the MHC class I cytoplasmic domain contains a tyrosine-based targeting signal required for routing MHC class I molecules through these compartments. We also report that transgenic mice expressing H-2Kb with a tyrosine mutation mount inferior H-2Kb-restricted cytotoxic T lymphocyte responses against two immunodominant viral epitopes, providing evidence of a crucial function for cross-priming in antiviral immunity.
Similar content being viewed by others
Main
Cell-mediated immunity against viral pathogens is dependent on specific priming of naive T lymphocytes by 'professional' antigen-presenting cells. DCs are the most potent antigen-presenting cells known, mainly because of their high surface expression of MHC class I and class II molecules and costimulatory molecules, and their ability to efficiently process a wide variety of potentially antigenic proteins into peptides for presentation to both CD4+ and CD8+ T cells1. Unlike most other cell types, DCs are able to present exogenously derived peptides in the context of MHC class I molecules2,3. Over the years, such cross-presentation has increasingly become recognized to be necessary, if not essential, for a wide variety of CD8+ T cell–mediated immune responses, including those to viruses, bacteria, allografts, tumor and self antigens4,5,6,7,8. Despite this, the mechanistic details underlying this alternative antigen-presentation pathway remain mostly undefined.
Evidence has supported two principal, nonmutually exclusive, models to explain how MHC class I molecules in DCs acquire exogenously derived peptide antigens. The first model, whose mechanism is dependent on the transporter associated with antigen processing (TAP), proposes that internalized antigens are normally routed from endolysosomal compartments directly into the cytosol, after which they follow the conventional pathway of proteosome digestion and TAP transport of peptides into the lumen of the endoplasmic reticulum9,10,11. Peptide binding to nascent MHC class I heavy chain and β2-microglobulin then facilitates conformational changes that allow for secretory transport of trimeric MHC class I complexes out of the endoplasmic reticulum, through the Golgi apparatus and to the cell surface. The second model (TAP-independent) proposes that MHC class I molecules encounter and bind exogenously derived peptides in post-Golgi or endolysosomal compartments, much as do MHC class II molecules, before being transported to the cell surface for presentation to specific CD8+ T cells12,13,14. Because the cytoplasmic domains of the invariant chain, MHC class II and CD1 molecules all contain endosomal trafficking motifs that direct their trafficking through endolysosomal compartments15,16 and ultimately their acquisition of exogenous antigens17,18, we sought to determine whether the MHC class I cytoplasmic tail had a similar function in antigen presentation.
Encoded by three separate exons (6, 7 and 8), the amino acid sequences of MHC class I cytoplasmic domains of evolutionarily distant species show a notable pattern of conservation (Fig. 1a) of one tyrosine residue (encoded by exon 6) and of two serine residues (encoded by exon 7), one of which is a known site of phosphorylation19. Splicing of exon 7 occurs naturally in some cell types20, resulting in MHC class I molecules that cannot be internalized efficiently in lymphoblastoid cell lines21.
(a) Aligned amino acid sequences of MHC class I cytoplasmic domains from 15 widely divergent vertebrate species. In bold, conserved tyrosine and serine residues. Species and MHC class I alleles (top to bottom): mouse (Mus musculus), H-2Kb; brown rat (Rattus norvegicus), RT1-AA; rabbit (Oryctolagus cuniculus), RLA 11/11; dog (Canis familiaris), DLA A9/A9; domestic cat (Felis catus), FLA-2; horse (Equus caballus), EX-MHC-B1; sheep (Ovis aries), SHP-MHCE; bull (Bos Taurus), BOLA BL3-6; human (Homo sapiens), HLA-A0101; harbour seal (Phoca vitulina), PLA-A1; chicken (Gallus gallus), B-FIV; African clawed frog (Xenopus laevis), Xen-MHC I; reptile (Ameiva ameiva), MHC I; leopard shark (Triakis scyllium), UAA; zebrafish (Brachydanio rerio), UBA-01. References for these amino acid sequences can be found in the Supplementary Note online. (b) Intron and exon structure of the gene encoding H-2Kb. The last three exons (6, 7 and 8) have been expanded to show the wild-type (WT) amino acid sequence of the cytoplasmic region of H-2Kb and the predicted amino acid sequences of the cytoplasmic H-2Kb mutants Δ7 and ΔY. S, signal sequence; TM, transmembrane domain. In bold, conserved tyrosine and serine residues. Upward arrow, single point mutation in ΔY (tyrosine to phenylalanine). (c) Pulse-chase experiment. Maturation rates of H-2Kb proteins are similar in splenic lymphocytes derived from transgenic mice expressing wild-type (WT) H-2Kb, ΔY or Δ7. H-2Kb proteins were immunoprecipitated with conformation-dependent antibody AF6-88.5 (at the times indicated above each lane) after pulse-labeling of cells with [35S]cysteine and methionine. The Δ7 molecule, predicted to lack 13 amino acids, shows the expected increased mobility by SDS-PAGE compared with that of wild-type H-2Kb and ΔY molecules.
Functional in vitro studies have shown the MHC class I cytoplasmic domain to be completely unnecessary for transport to the cell surface as well as for the ability of MHC class I molecules to acquire antigens through the conventional pathway and act as targets for allogeneic or specific cytotoxic T lymphocytes (CTLs)22,23. However, the sole report to our knowledge investigating the function of the cytoplasmic domain in the natural generation of MHC class I–restricted immune responses showed that transgenic mice expressing a glycosyl-phosphatidylinositol–anchored form of H-2Db failed to mount an effective CTL response to an immunodominant H-2Db-restricted influenza A virus epitope24. This result has indicated that the cytoplasmic and/or transmembrane MHC class I domains are much more important in vivo.
Our study here extends these findings with the demonstration that the highly conserved MHC class I cytoplasmic tyrosine residue forms part of an intracellular targeting motif that is responsible for MHC class I endolysosomal trafficking in DCs leading to the acquisition and subsequent cross-presentation of exogenously derived peptide antigens. Furthermore, we show that transgenic mice expressing H-2Kb molecules with a tyrosine point mutation generate inferior CTL responses against two separate H-2Kb-restricted immunodominant viral epitopes, providing evidence that cross-presentation forms a crucial component of antiviral immunity in vivo.
Results
H-2Kb mutagenesis and generation of transgenic mice
To explore whether the MHC class I cytoplasmic tail has a function in antigen presentation, we first used site-directed mutagenesis to introduce specific mutations into the mouse H-2Kb genetic sequence that encodes the cytoplasmic domain. Two mutants were generated (Fig. 1b): H-2Kb mutant Δ7 contains a complete deletion of exon 7 (encoding 13 amino acids of the tail, including the serine phosphorylation site); and mutant ΔY contains a single point mutation substituting a phenylalanine residue for the conserved tyrosine residue of exon 6. We sequenced these mutated gene constructs and the gene encoding wild-type H-2Kb and microinjected them separately into fertilized mouse oocytes to generate transgenic mouse lines expressing each of the H-2Kb transgenes. We bred transgenic mice onto an H-2k background by backcrossing transgenic founders with C3H/He mice for a minimum of six generations. We also transfected mouse L cell fibroblasts (H-2k) with the same H-2Kb DNA constructs.
Protein analysis showed that the Δ7 and ΔY mutant H-2Kb molecules had the predicted molecular weight based on the mutations made (Fig. 1c). Pulse-chase experiments of spleen-derived lymphocytes showed that Δ7 and ΔY molecules matured at rates similar to those of wild-type H-2Kb molecules in both wild-type H-2Kb transgenic and C57BL/6 control mice, as determined by their acquisition of glycosylation groups and resistance to endo-N-acetylglucoseaminidase H over time (Fig. 1c and data not shown). These data indicated that in lymphocytes, transport of nascent MHC class I complexes from the endoplasmic reticulum through the Golgi and to the cell surface was not affected by the cytoplasmic mutations. We obtained similar results with transfected fibroblasts (data not shown).
CTL responses in vivo require cytoplasmic tyrosine
To determine the function of the MHC class I cytoplasmic domain in vitro and in vivo, we evaluated viral antigen presentation both in cultured fibroblasts and in transgenic mice. As predicted, L cell fibroblast transfectants expressing similar amounts of wild-type, ΔY or Δ7 H-2Kb (Fig. 2a) showed similar abilities to act as targets for CTLs specific for a single viral peptide consisting of amino acids 52–59 of the vesicular stomatitis virus (VSV) nucleocapsid protein (called VSV N(52–59) here), either when pulsed with VSV N(52–59) or when infected with VSV for 6, 12 or 18 h (Fig. 2b,c and data not shown). This result confirms previous studies22,23 and demonstrates that classical endogenous antigen processing and presentation in cultured fibroblasts is not affected by mutations to conserved regions of the MHC class I cytoplasmic tail.
(a) Surface expression of H-2Kb molecules in transfected L cell fibroblasts, as measured by flow cytometry. U, untransfected L cells. Numbers in histograms indicate mean fluorescence intensities. (b,c) Fibroblasts were either pulsed with VSV N(52–59) (1 μM; + Pep; b) or infected with VSV (+VSV; c) for 18 h before being used as targets for VSV N(52–59)–specific CTLs in a standard 51Cr-release assay. −Pep (b), unpulsed; −VSV (c), uninfected. (d) Surface expression of H-2Kb molecules in peripheral blood lymphocytes (PBLs) from transgenic mice expressing H-2Kb, as measured by flow cytometry. Numbers in histograms indicate mean fluorescence intensities. C3H, negative control mice. (e) Spleens from transgenic and control (C57) mice 6 d after intraperitoneal injection with VSV. Data represent spleen weights and splenocyte counts with (light gray and filled bars, respectively) or without (open and dark grey, respectively) VSV infection. (f–i) Antiviral CTL responses in transgenic mice expressing H-2Kb: At 6 d after intraperitoneal injection with infectious VSV (f), heat-inactivated VSV (g), infectious Sendai virus (h) or heat-inactivated Sendai virus (i), splenocytes were restimulated in culture for 5–6 d with 1 μM of the appropriate H-2Kb-restricted peptide: VSV N(52–59) or Sendai NP(324–332). Specific CTL activity was measured with a standard cytotoxicity assay, with 51Cr-labeled (H-2Kb-transfected L cell fibroblasts pulsed with the appropriate peptide as target cells. C3H, negative control mice; C57, positive control mice. Data represent 51Cr release (mean and s.d.) of two mice per group, with all measurements obtained in triplicate. Data are representative of at least three separate experiments. E:T, effector/target ratio; WT, wild-type.
The functional consequences of the H-2Kb cytoplasmic tail mutations were apparent only when we examined H-2Kb-restricted antiviral responses in vivo. Transgenic mice expressing similar amounts of wild-type H-2Kb, Δ7 and ΔY on peripheral blood leukocytes (Fig. 2d) generated notably dissimilar immune responses to viral infections. Whereas spleens from transgenic mice expressing wild-type H-2Kb or Δ7 and C57BL/6 control mice were visibly enlarged 5–6 d after intraperitoneal injection of VSV, spleens from VSV-infected mice expressing ΔY had much lower spleen weights and splenocyte counts (Fig. 2e), indicative of a diminished response to VSV infection. Although C3H/He mice (H-2k transgenic background) do not generate a detectable CTL response against VSV25, H-2Kb-expressing mice normally generate a vigorous, immunodominant response against VSV N(52–59)26. Consistent with this, splenocytes from VSV-infected wild-type H-2Kb and Δ7 mice demonstrated strong VSV nucleocapsid peptide–specific lytic activity after 5 d of in vitro peptide stimulation. In contrast, CTLs derived from ΔY mice typically required 10- to 20-fold higher effector/target ratios to achieve comparable lysis of 51Cr-labeled nucleocapsid peptide–pulsed target cells (Fig. 2f). We obtained similar results as early as 6 d after VSV infection, at which time anti-VSV CTL activity was detectable in splenocytes from both wild-type H-2Kb and Δ7 transgenic mice, but not in those of ΔY mice (data not shown).
To confirm this apparent deficiency in CTL priming with another viral system, we assessed CTL responses against Sendai virus in the H-2Kb-expressing transgenic mice. Sendai virus has the unique property of eliciting a H-2Kb-restricted CTL response when delivered in either an infectious or a heat-denatured form27, a property not shared with VSV (Fig. 2g). As with VSV, ΔY-expressing mice infected with infectious Sendai virus mounted a much weaker CTL response against the immunodominant H-2Kb-restricted Sendai nucleoprotein epitope consisting of amino acids 324–332 (called NP(324–332) here) than did transgenic mice expressing wild-type H-2Kb or Δ7 and C57BL/6 control mice (Fig. 2h). This defect was greatest in ΔY mice immunized with heat-denatured Sendai virus (Fig. 2i). Because this last antigen must generate CTL solely through cross-presentation, this result indicated that the antigen-presenting cells of the ΔY strain had lost the capacity to present exogenous (nonreplicative) Sendai virus antigen in the context of H-2Kb molecules.
DC cross-presentation requires cytoplasmic tyrosine
We next evaluated whether inefficient cross-presentation by DCs may be responsible for the lack of H-2Kb-restricted antiviral CTL activity in ΔY transgenic mice. We examined bone marrow–derived and spleen-derived DCs from the different transgenic mouse strains for their ability to cross-present the well characterized H-2Kb-restricted ovalbumin epitope consisting of amino acids 257–264, OVA(257–264)10. We isolated immature bone marrow DCs and differentiated them in culture with granulocyte-monocyte colony-stimulating factor (GM-CSF) as described28. After 16 h of incubation with soluble whole ovalbumin at a concentration of 5 mg/ml, we exposed DCs to B3Z, a T cell hybridoma that is activated by the recognition of H-2Kb in association with OVA(257–264)29. DCs derived from transgenic mice expressing wild-type H-2Kb or Δ7 efficiently activated B3Z cells after incubation with soluble ovalbumin, as did EG7 control cells (EL4 cells transfected with ovalbumin cDNA). However, we found no activation of B3Z cells after incubating them with ovalbumin-loaded DCs derived from transgenic mice expressing ΔY (Fig. 3a).
(a) Activation of B3Z T cell hybridoma by immature bone marrow–derived DCs after 16 h incubation with (filled bars) or without (open bars) soluble ovalbumin (5 mg/ml). Activation of B3Z was measured with a chemiluminescence assay system to detect interleukin 2 promotor–driven β-galactosidase (lacZ) production. EG7, control cells (EL4 cells transfected with ovalbumin cDNA). Data represent means ± s.d. of results obtained from triplicate wells. Similar results were obtained in three separate experiments. (b) Presentation of exogenous ovalbumin by spleen-derived DCs, as measured by B3Z activation. Data represent means ± s.d. of results obtained from triplicate wells. Similar results were obtained in three different experiments. (c) Presentation of exogenous ovalbumin by splenic DCs, as measured by specific CTL lysis. CD11c+ cells purified from spleens of H-2Kb transgenic mice were incubated with ovalbumin, labeled with 51Cr and exposed to activated OT-1 (H-2Kb-OVA-specific) CTLs at various effector/target ratios (E:T; horizontal axis). Data represent the means ± s.d. of specific 51Cr release as measured in triplicate wells. Similar results were obtained in two separate experiments. (d) Presentation of H-2Kb–OVA(257–264) complexes by splenic DCs expressing similar amounts of surface H-2Kb after overnight incubation with soluble ovalbumin (10 mg/ml), infection with vaccinia virus encoding ovalbumin (MOI 5) or vaccinia virus encoding OVA(257–264) (MOI 5), or incubation with synthetic OVA(257–264) (20 μg/ml). DCs were stained with mAb 25.D1.16, specific for H-2Kb–OVA(257–264) complexes. DCs pulsed with an irrelevant protein (BSA) or peptide, or infected with an empty vaccinia virus, were used as negative controls (blue). Data are representative of three separate experiments. P < 0.05, all histogram peak shifts except for ovalbumin protein–pulsed ΔY-derived DCs (comparison of mean fluorescence intensities over the three experiments; Z-test, two-sided). WT, wild-type.
We also isolated DCs from the spleens of all transgenic mice with CD11c+ magnetic beads before incubating the cells overnight with soluble ovalbumin at various concentrations and exposing them to B3Z cells. Again, DCs derived from transgenic mice expressing ΔY were considerably less efficient at activating B3Z cells than were those from transgenic mice expressing wild-type H-2Kb (Fig. 3b). DCs derived from Δ7 mice elicited intermediate B3Z reactivity in these experimental conditions. We obtained similar results with a chromium-release assay to measure cytolysis of ovalbumin-pulsed DCs by activated CTL derived from OT-I mice that express a transgenic TCR specific for H-2Kb–OVA(257–264) complexes30 (Fig. 3c).
To quantify cross-presentation more directly, we assessed DCs with a monoclonal antibody (mAb 25.D1.16) that specifically recognizes H-2Kb–OVA(257–264) complexes31. Expression of these complexes on the surfaces of DCs derived from both wild-type H-2Kb and Δ7 mice was measurable with 25.D1.16 after overnight exposure to soluble ovalbumin (Fig. 3d). No H-2Kb–OVA(257–264) complexes were detectable, however, in DCs derived from transgenic mice expressing ΔY. Infection of these same DCs with a vaccinia virus expressing either whole ovalbumin protein or a 'minigene' encoding OVA(257–264)32 showed that endogenous antigen presentation was intact and nearly equivalent in DCs from all three mouse strains (Fig. 3d). These experiments clearly demonstrated that the cytoplasmic tyrosine point mutation had abrogated the ability of H-2Kb molecules specifically to cross-present the ovalbumin epitope OVA(257–264) in both bone marrow- and spleen-derived DCs.
Cytoplasmic tyrosine controls endolysosomal trafficking
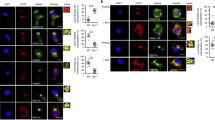
To better understand this deficiency in cross-presentation, we used comparative immunofluorescence confocal microscopy to determine the intracellular localization of wild-type H-2Kb, Δ7 and ΔY molecules in bone marrow- and spleen-derived DCs. We generated transgenic bone marrow DCs as described28 and collected them on day 9 to obtain a population of intermediate stage DCs that was >80% pure (Fig. 4a). We stained cells with conformation-dependent mAbs specific for H-2Kb or H-2Kk in addition to a mAb specific for the early endosome marker EEA-1. Immunofluorescence confocal microscopy showed a morphology typical of intermediate-stage DCs, with most cells having a rounded shape lacking cytoplasmic processes. H-2Kk molecules, endogenously expressed by all our transgenic mouse strains, localized at or near the cell surface in most cells examined (Fig. 4b–d). Similarly, most wild-type H-2Kb molecules were localized at the surface or inside vesicles near the cell surface (Fig. 4e). Both Δ7 and ΔY molecules, however, showed altered intracellular distributions compared with those of wild-type MHC class I molecules. The Δ7 molecules showed some surface distribution, but a substantial portion of the molecules localized to a single intracellular compartment (Fig. 4f). The ΔY molecules had an even greater redistribution to a single intracellular compartment in most cells examined, with a distinct down-regulation of surface staining (Fig. 4g). We found little or no colocalization of MHC class I molecules with EEA-1, providing further evidence that for intermediate stage DCs, MHC class I molecules reside in a compartment that is distinct from recycling early endosomes33.
Intermediate-phenotype bone marrow DCs were collected on day 9 and cultured overnight on coated coverslips before being washed, fixed and permeabilized. (a) Histograms show intermediate DC phenotype. Red, specific staining for DC surface molecules; blue, isotype control mAb staining. (b–g) Cells were stained with an H-2Kk-specific mAb (b–d; green) or an H-2Kb-specific mAb (e–g; green), in addition to a mAb specific for EEA-1, a marker of early endosomes (red). These optically merged images are representative of most cells examined by immunofluorescent confocal microscopy. Scale bars represent 10 μm.
We next examined spleen-derived DCs by immunofluorescence confocal microscopy to determine whether localization of Δ7 and ΔY molecules had been similarly altered and to identify the intracellular compartments where the molecules were localized at later stages of maturation. We isolated splenic DCs and cultured them for 48 h in the presence of GM-CSF and tumor necrosis factor-α to obtain a homogeneous population of mature DCs (Fig. 5a). Nonadherent cells were effectively enriched for DEC-205+ cells, the DC subset shown to be the most efficient at cross-presentation34,35. We examined the three main known compartments of MHC class I trafficking (endoplasmic reticulum, Golgi apparatus and endolysosomes) for colocalization with MHC class I molecules. Cell morphologies of DCs were typical of the late stage of maturation, with most cells demonstrating dendritic processes in all populations examined. We noted little colocalization of MHC class I molecules with the endoplasmic reticulum marker GRP78 for any of the cells examined (data not shown), indicating that transport from endoplasmic reticulum to Golgi in DCs is independent of the MHC class I cytoplasmic tail, as has been described for other cell types22. Double-labeling for H-2Kb and the Golgi marker Giantin, however, showed that although there was no notable colocalization for wild-type H-2Kb and Δ7 molecules (Fig. 5b,c), ΔY molecules colocalized extensively with Giantin in approximately 70% of DCs examined (Fig. 5d,e).
Mature phenotype splenic DCs were purified from transgenic mouse spleens with CD11c magnetic beads, cultured, plated on coverslips, fixed and permeabilized. (a) Histograms show intermediate DC phenotype. Red, specific staining for DC surface molecules; blue, isotype control mAb staining. (b–i) Cells were stained with an H-2Kb-specific mAb (green) and either a mAb specific for the cis-Golgi marker Giantin (b–e; red) or a mAb specific for the endolysosomal marker Lamp1 (f–i; red). These optically merged images are representative of most cells examined by immunofluorescent confocal microscopy. Yellow, colocalization of green and red. Scale bar represents 10 μm.
We found extensive colocalization of wild-type H-2Kb molecules with the transmembrane lysosomal protein Lamp1 in nearly all DCs examined (Fig. 5f), confirming the findings of others that MHC class I molecules normally reside in and traffic through endolysosomal compartments of DCs33. Similarly, Δ7 molecules demonstrated an intense overlap with Lamp1-positive compartments in nearly all DCs examined (Fig. 5g). In contrast, ΔY molecules showed only a modest degree of colocalization with Lamp1 in <25% of DCs, with the remainder demonstrating a complete lack of endolysosomal localization (Fig. 5h,i). Thus, the cytoplasmic tyrosine point mutation resulted in a considerable misrouting of MHC class I molecules, with only a fraction of molecules being targeted to endolysosomal compartments in most mature DCs. This misrouting did not alter surface expression of ΔY molecules appreciably, as H-2Kb expression in these DCs was similar to those of wild-type H-2Kb and Δ7 DCs, as determined by flow cytometry (Fig. 3d).
Exogenous peptide loading within DC endolysosomes
To identify the compartments in which H-2Kb–OVA(257–264) peptide complexes were localized within splenic DCs after overnight incubation with whole ovalbumin protein, we used intracellular staining with mAb 25.D1.16. We found no colocalization of H-2Kb–OVA(257–264) complexes with the endoplasmic reticulum marker GRP78 for any of the transgenic mouse–derived DCs (data not shown). Similarly, in DCs derived from wild-type H-2Kb and Δ7 mice, we found no colocalization of these complexes with the Golgi marker Giantin (Fig. 6a,b). However, DCs derived from ΔY mice demonstrated relatively sparse specific staining for H-2Kb–OVA(257–264), and the few complexes present were exclusively within Golgi compartments (Fig. 6c).
After overnight incubation with ovalbumin (10 mg/ml), mature spleen-derived DCs were stained with a mAb specific for H-2Kb–OVA(257–264) complexes (red) and counterstained with either a mAb to the cis-Golgi marker Giantin (a–c; green) or a mAb specific for the endolysosomal marker Lamp1 (d–f; green). These optically merged images are representative of most of the cells examined by immunofluorescence confocal microscopy. Yellow, colocalization of green and red. Scale bar represents 10 μm.
Of the DCs derived from wild-type H-2Kb and Δ7 spleens staining positive for H-2Kb–OVA(257–264) complexes, >80% demonstrated strong colocalization with Lamp1 (Fig. 6d,e). In contrast, we found no H-2Kb–OVA(257–264) complexes in Lamp1-positive compartments in DCs expressing ΔY molecules (Fig. 6f). These results indicate that endolysosomal (Lamp1-positive) compartments of DCs comprise the main sites for MHC class I loading of cross-presented ovalbumin peptide, and provide an explanation for the inability of H-2Kb molecules with mutated tyrosine to efficiently cross-present this epitope.
Discussion
The results presented here provide evidence that the MHC class I cytoplasmic tail has a functional involvement in antigen presentation. Most previous studies designed to assess the function of the MHC class I cytoplasmic domain used in vitro systems and examined only the classical endogenous antigen-presentation pathway22,23. Our findings confirm those results and extend those studies with the use of a mouse model to evaluate the function of the MHC class I cytoplasmic domain in vivo.
The conserved MHC class I cytoplasmic tyrosine residue seemed to be pivotal for proper endolysosomal targeting in DCs and subsequent cross-presentation of exogenously derived antigens. Because DCs are unique in their ability to efficiently cross-present antigens to activate naive CD8+ T cells, this indicates that the tyrosine-based endolysosomal targeting signal may be specific for DCs. Many other membrane proteins are dependent on cytoplasmic YXXØ (where Ø is a bulky, hydrophobic amino acid) tyrosine-based trafficking motifs for targeting to lysosomes36,37. Because the third amino acid position after the MHC class I cytoplasmic tyrosine residue (alanine) is also highly conserved, it is possible that the YXXA motif forms a trafficking signal analogous to those of other membrane proteins, although this remains to be demonstrated. CD1b and CD1d molecules provide relevant examples, as these nonclassical MHC class I molecules are dependent on a cytoplasmic tyrosine motif for their targeting to endolysosomal (Lamp1-positive) compartments, where they have been shown to acquire glycolipid antigens before mobilizing to the cell surface for presentation to natural killer T cells16,17. Adaptin proteins specifically recognize and bind to tyrosine motifs as the first step in the formation of coated transport vesicles38. DC-specific adaptin proteins may normally serve to link the MHC class I cytoplasmic tail to the endolysosomal vesicular transport pathway, and substitution of the single cytoplasmic tyrosine residue may be able to effectively abrogate this interaction39.
Our data here support a model in DCs in which the cytoplasmic tyrosine motif normally targets a subset of MHC class I molecules to endolysosomal compartments, where they undergo peptide exchange and acquire exogenously derived antigens before mobilizing to the cell surface. The ultimate source of MHC class I molecules in these compartments remains to be determined, but recycling from the cell surface is a strong possibility, as both exon 7 and the cytoplasmic tyrosine residue regulate MHC class I endocytosis in lymphoblastoid cell lines21,40. Studies of a DC-like cell line have shown evidence of resident MHC class I molecules in the Golgi compartments of immature cells, but these have been shown to mobilize directly to the cell surface after maturation41. The consistently high surface expression of ΔY molecules found in mature DCs indicates that this mobilization to the cell surface is generally intact. However, our finding of some ΔY molecules in the Golgi compartments of mature DCs indicates that a subset of MHC class I molecules may normally be sorted into endolysosome-bound vesicles directly from the Golgi. In the absence of a tyrosine motif, these molecules may be trapped in Golgi compartments en route to endolysosomes.
The small number of surface H-2Kb–OVA(257–264) complexes produced by cross-presentation compared with direct presentation probably reflects differences in the relative efficiencies of the two pathways and indicates that cross-presentation involves only a fraction of the total MHC class I pool present in DCs. Nonetheless, this modest amount of peptide presentation is immunologically relevant, as demonstrated by the considerable specific activation of T cells recognizing H-2Kb–OVA(257–264) complexes. The observation that H-2Kb–OVA(257–264) complexes were undetectable with mAb on the surface of DCs derived from ΔY mice, yet still activated T cells specific for H-2Kb–OVA(257–264) at a low level confirms that T cells are responsive to very small amounts of MHC class I–peptide complexes and that T cell recognition provides a much more sensitive, albeit less quantitative, assay for peptide presentation31. This acquisition of OVA(257–264) peptide by ΔY molecules may reflect endosome-to-cytosol transport of ovalbumin and subsequent loading of these MHC class I molecules in the endoplasmic reticulum through the classical endogenous antigen-presentation pathway. Alternatively, it may reflect a 'leakiness' of ΔY molecules to traffic through Lamp1-positive compartments. This latter possibility would not be unlikely, given the extremely conservative nature of the ΔY mutation (removal of a single oxygen atom) as well as the fact that tyrosine-based endosomal trafficking motifs do not seem to involve direct tyrosine phosphorylation39.
The unexpected discovery that a point substitution to the cytoplasmic MHC class I tyrosine residue specifically abrogates cross- presentation in DCs and also impairs CD8+ T cell priming in response to two separate viruses raises many immunological implications. Visualization of wild-type H-2Kb–OVA(257–264) complexes exclusively in Lamp1-positive compartments of DCs after incubation with ovalbumin protein provides evidence that these compartments comprise a chief site of exogenous antigen loading for MHC class I molecules. This evidence is strengthened by the fact that ΔY molecules, lacking the conserved cytoplasmic tyrosine residue and unable to traffic through these compartments, were substantially impaired in their ability to acquire and subsequently present the OVA(257–264) peptide at the DC surface.
The demonstration that ΔY molecules were able to efficiently acquire and present two different endogenously synthesized peptides (in VSV-infected fibroblasts and in DCs infected with vaccinia virus–ovalbumin), but were unable to effectively cross-present two exogenous antigens (DCs pulsed with whole ovalbumin and CTL priming in vivo with heat-inactivated Sendai virus) shows that only cross- presentation, and not direct presentation, was affected by the tyrosine point mutation. Furthermore, the observation that mice expressing ΔY molecules were substantially impaired in their ability to generate CTL responses against two infectious viruses generating well- characterized, H-2Kb-restricted immunodominant epitopes (VSV and Sendai virus) supports previous studies indicating that cross- presentation may be the main mechanism by which antiviral CD8+ T cell-mediated immunity is generated naturally in vivo4.
The source of antigenic peptides remains a central question in cross-presentation. Peptides capable of binding to MHC class I molecules may be generated directly within endolysosomal compartments by cathepsins and other proteolytic enzymes. Internalized ovalbumin, however, is transported efficiently into the cytosol of DCs through an endosome-to-cytosol route10,42. Further evidence of a cytosolic component to ovalbumin processing comes from studies showing that cross-presentation of OVA(257–264) is subject to proteosomal blockade43,44. Given those results, our data support a third, alternative model to explain cross-presentation, which incorporates the phenomenon of endoplasmic reticulum–mediated phagocytosis in macrophages45,46. This model proposes the existence of phagosomal compartments, generated through fusion of the endoplasmic reticulum with endolysosomal vesicles47, which are positive for both Lamp1 and TAP. Similar fusion events of the endocytic pathway with the classical endogenous antigen-presentation machinery of DCs could explain many aspects of cross-presentation, including its dependence on proteosomes and TAP, as well as many of our results. This model does not, however, fully explain the apparent sequestration of exogenously acquired ovalbumin from endogenously synthesized ovalbumin demonstrated in our ΔY model. Although much remains to be determined regarding the nature of endolysosomal antigen processing, the identification of an MHC class I endolysosomal targeting motif provides a critical step in delineating the mechanisms behind cross-presentation.
The human immunodeficiency virus 1 protein Nef causes down-regulation of MHC class I molecules by sequestering them in Golgi compartments, a property that is directly dependent on the MHC class I cytoplasmic tyrosine residue48. Our results raise the possibility that Nef interferes with the same pathway that normally controls MHC class I endolysosomal targeting in DCs. Because DCs are a known reservoir of human immunodeficiency virus, infected DCs may be particularly susceptible to MHC class I misrouting by Nef49, which in vivo could lead to a deficiency in DC cross-presentation of opportunistic pathogen-associated antigens.
These results provide a compelling justification for the high level of conservation shown by the MHC class I cytoplasmic tyrosine motif, an evolutionary event that itself may emphasize the importance of cross-presentation in vivo. Furthermore, the establishment of this model should now allow for direct assessment of the function of cross- priming in fundamental immunological events such as tolerance to self and tumors, and the generation of autoimmune responses.
Methods
Mutagenesis of the gene encoding H-2Kb.
Mutations were introduced into the mouse gene encoding H-2Kb with a PCR-based protocol50. The primers containing mismatches and deletions used for generating the mutant constructs were as follows. For the tyrosine-to-phenylalanine codon substitution in exon 6 (underlined) at position 321 (ΔY mutant), 5′-AAAAGGAGGGGACTTCGCTCTG GCTCCA-3′ was used, and for the complete deletion of exon 7 (residues 326–338; Δ7 mutant), 5′-TTTGTTTTGTTTTACC:TGACACTCTAGGGTCT-3′ was used (colon, location of deleted sequence). The 'bookend' primers50 used for the mutation of exon 6 (Y→F) were 5′-TCTGAGTTTTCTCTCAGCCTCCTTTAGAGTGT-3′ and 5′-TAAAACAAAACAAATGTATATGCATATGTACAT-3′. The bookend primers used for the deletion of exon 7 were 5′-ACATTGGAGTGAAGTTGGAGATGATGGGA-3′ and 5′-AACATCAGACACTTGACTAAAGAGAA CT-3′. PCR reactions consisted of 30 cycles of denaturation at 95 °C for 30 s followed by annealing and extension at 60 °C for 60 s, with 0.25 μM primers, 200 μM dNTPs and Pfu polymerase (Stratagene) in the manufacturer's buffer. PCR fragments were digested with restriction enzymes and the cut fragments were gel-purified and ligated into the linearized pKb vector. The validity of these constructions was confirmed by DNA sequencing.
Transgenic mice.
The DNA constructs described above were purified on cesium chloride gradients, and the genes encoding wild-type and mutant H-2Kb were subsequently excised from their original cloning vector, pBR322, by an EcoRI digestion followed by agarose gel purification. DNA was diluted to a concentration of 1 μg/ml and was microinjected into fertilized mouse oocytes (derived from a C3H/He × C57BL/6 mating), which were then transplanted into the uteri of pseudo-pregnant female mice. Transgenic offspring were originally selected by Southern blot analysis. These founder mice were tested for increased peripheral blood leukocyte H-2Kb expression by flow cytometry compared with that of H-2b/k control mice, indicative of transgene expression. Three founder mice from each group were chosen and subsequently bred to C3H/He mates for a minimum of six generations. All transgenic mouse studies were approved by the Committee on Animal Care at the University of British Columbia using the guidelines set out by the Canadian Council on Animal Care.
Peripheral blood leukocyte isolation and flow cytometry.
Peripheral blood leukocytes were obtained from mice by a tail bleed. Blood (50–100 μl) was initially mixed with 1 M heparin (75 μl) and then PBS (825 μl) before being separated on Ficoll gradients by centrifugation. AF6.88.5, 28.14.8S and 16.3.1N conformation-dependent mouse mAbs (of the IgG2a isotype) were specific for extracellular domains of H-2Kb, H-2Db and H-2Kk, respectively. Cells (1 × 105 to 2 × 105) were stained with the appropriate MHC class I–specific primary antibody and then were incubated with goat antibody to mouse IgG2a (anti-IgG2a) conjugated to biotin (Southern Biotechnology) followed by streptavidin–fluorescein isothiocyanate (Jackson ImmunoResearch Laboratories). Stained peripheral blood leukocytes were fixed, and leukocyte-gated cells were analyzed by flow cytometry (Becton Dickinson).
Immunoprecipitation.
Spleen-derived lymphocytes were incubated for 20 min in methionine- and cysteine-free media, pulsed for 20 min with [35S]methionine (300 uCi/ml), and chased in medium containing excess methionine (0.5 mM) and cysteine (0.5 mM). At various time points, lymphocytes (1 × 107) were collected, washed in cold PBS and lysed for 20 min at 4 °C in 1% Nonidet-P40 buffer (120 mM NaCl, 4 mM MgCl2 and 20 mM Tris-HCl, pH 7.6) containing phenylmethyl sulfonyl fluoride (40 μg/ml). After cell nuclei had been centrifuged, cell lysates were precipitated with trichloracetic acid and samples with equal amounts of incorporated radioactivity were immunoprecipitated with mAb AF6.88.5. Proteins were separated on a 10–15% gradient gel in reducing conditions by SDS-PAGE. The gel was then fixed, enhanced, dried and exposed to autoradiographic film.
Generation of virus-specific CTLs.
VSV- or Sendai virus–specific CTLs were generated by in vivo priming followed by in vitro restimulation. Mice were infected by intraperitoneal injection of virus: VSV (1 × 107 '50% tissue culture–infectious dose', or TCID50) per mouse (or 3 × 107 TCID50 per mouse for heat-inactivated VSV); Sendai virus (1.5 × 107 '50% egg-infectious dose units', or EID50) per mouse (or 5 × 107 EID50 per mouse for heat-inactivated Sendai). The TCID50 and EID50 are the endpoint dilutions of the virus that infect 50% of inoculated tissue culture cells and embryonated chicken eggs, respectively. Heat inactivation of viruses was accomplished by incubation of virus preparations for 2 h at 65 °C before infection of mice. Heat inactivation was confirmed experimentally by infection of susceptible target cells. At 6 d after viral infection, mice were killed and spleens were collected. Splenocytes were cultured at a density of 2.5 × 106 cells/ml for 5 d in RPMI 1640 medium supplemented with 10% FCS (HiClone), nonessential amino acids (0.1 mM), sodium pyruvate (1 mM), 2-mercaptoethanol (50 μM), L-glutamine (2 mM) and 1 μM of the appropriate H-2Kb-restricted peptide: VSV N(52–59), RGYVYQGL, or Sendai NP(324–332), FAPGNYPAL.
Antiviral cytotoxicity assays.
Cytotoxicity was assessed with a standard 51Cr-release assay. Target cells (H-2Kb-transfected L cell fibroblasts) were incubated with 1 μM peptide and labeled for 1.5 h with sodium chromate (100 μCi; Amersham), then washed and resuspended in RPMI 1640 complete medium. Effector cells were incubated for 4 h at 37 °C with target cells (1 × 104 cells per well in 96-well plates) at various effector/target ratios. Spontaneous 51Cr release by labeled cells was measured in the absence of CTL, and maximum release was quantified by lysis of target cells in 2.5% Triton X-100 detergent. All experiments were done in triplicate, and specific 51Cr release was calculated with the following equation: Percent specific 51Cr release = [(experimental release – spontaneous release) / (maximum release – spontaneous release)] × 100%. Spontaneous background 51Cr release never exceeded 15% of maximum release.
DC culture.
DCs were derived from mouse bone marrow precursors by culture in vitro with GM-CSF (20 ng/ml) as described28. Bone marrow DCs were collected on day 7 and day 9 to obtain immature and intermediate stage DC populations, respectively. Splenic DCs were isolated with CD11c magnetic beads and were either used immediately or cultured for 48 h in GM-CSF and tumor necrosis factor-α (10 ng/ml) to induce full maturation. Nonadherent splenic DCs were used, providing for the enrichment of DEC-205+ DC subsets capable of efficient cross-presentation34,35. All DCs were analyzed by flow cytometry for cell surface expression of CD11c, DEC-205, CD40, CD86 and I-Ak to assess purity and stage of maturation.
Detection of cross-presentation of ovalbumin.
B3Z cells express a TCR that specifically recognizes OVA(257–264) (SIINFEKL) in the context of H-2Kb in addition to carrying a β-galactosidase (lacZ) construct driven by nuclear factor of activated T cells elements from the interleukin 2 promoter29. An assay with Galacton-Star as a chemiluminescent substrate was used for the detection of lacZ activity in B3Z lysates. Bone marrow DCs (day 7, immature phenotype) were incubated for 16 h with whole ovalbumin (5 mg/ml) before being washed and incubated overnight with B3Z cells at a cell ratio of 1:1. DCs were also isolated from the spleens of transgenic mice expressing H-2Kb with CD11c+ magnetic beads (Miltenyi Biotech), and were exposed to varying concentrations of ovalbumin for 16 h before being incubated with B3Z cells as described above. OT-I mice express a transgenic TCR that specifically recognizes H-2Kb in complex with OVA(257–264)30. For the generation of CTLs specific for H-2Kb–OVA, 1 × 107 EG7 cells (EL4 cells transfected with ovalbumin cDNA) were irradiated (20,000 rad) and cultured for 6 d in complete RPMI 1640 medium with 1 × 107 spleen cells from OT-I mice. Spleen-derived DCs were incubated for 16 h with ovalbumin (5 mg/ml) and labeled for 1.5 h with sodium chromate (51Cr) in RPMI 1640 medium before being exposed to OT-I effector CTLs for 4 h at 37 °C. The percentage of specific lysis was calculated as described above. After overnight incubation with 5 mg/ml of soluble ovalbumin or control antigen BSA, Fc receptors of splenic DCs were blocked with 2.4G2 FcγIII/II blocker (PharMingen), then cells were stained with mAb 25.D1.16, specific for H-2Kb–OVA(257–264) complexes31, or IgG1 isotype control antibody followed by phycoerythrin-conjugated rat anti-mouse IgG1. DCs were then washed and analyzed by flow cytometry.
Infection of DCs.
Splenic DCs were isolated from all mouse strains as described above and infected at a multiplicity of infection (MOI) of 5 for 2 h with either empty recombinant vaccinia virus, recombinant vaccinia virus expressing full-length ovalbumin or recombinant vaccinia virus expressing a 'minigene' encoding OVA(257–264)32. After culture in complete RPMI medium for 16 h, Fc receptors were blocked in DCs and cells were stained with mAb 25.D1.16 or IgG1 isotype control antibody, followed by phycoerythrin-conjugated rat anti-mouse IgG1. DCs were then washed and analyzed by flow cytometry.
Confocal microscopy.
Bone marrow– and spleen-derived DCs were isolated as described above. Bone marrow–derived DCs with an intermediate phenotype (day 9) were isolated and plated on polylysine-coated coverslips for 16 h. Next, cells were washed with PBS, fixed with paraformaldehyde (2%) and permeabilized with saponin (0.1%). DCs were double-stained with mAb AF6-88.5 (anti-H-2Kb) or mAb 36-7-5 (anti-H-2Kk; PharMingen) and a rabbit mAb specific for EEA-1, a marker of early endosomes. Spleen-derived CD11c+ cells with a mature phenotype were plated overnight on coverslips, fixed and permeabilized as described above, and double-stained with mAb AF6-88.5 and Giantin (Calbiochem) or Lamp1 (Santa Cruz Biotechnology) rabbit mAbs. Secondary Alexa-488 green–conjugated goat anti-mouse and Alexa-568 red–conjugated goat anti-rabbit (Molecular Probes) were used as detection reagents. Alternatively, mature DCs were stained with conjugated mAb 25.D1.16 to detect H-2Kb–OVA(257–264) complexes after incubation with soluble ovalbumin (10 mg/ml) or control protein antigen BSA. Isotype control antibodies were used in all confocal microscopy experiments to confirm specificity of antibody stainings. Coverslips were examined by confocal microscopy, and Z-series' of multiple images were acquired from DCs representative of most cells in each culture. Figures are shown as optically merged 'green-plus-red' images.
Note: Supplementary information is available on the Nature Immunology website.
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Yewdell, J.W., Norbury, C.C. & Bennink, J.R. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73, 1–77 (1999).
Heath, W.R. & Carbone, F.R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Sigal, L.J., Crotty, S., Andino, R. & Rock, K.L. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398, 77–80 (1999).
Svensson, M. & Wick, M.J. Classical class I peptide presentation of a bacterial fusion protein by bone marrow-derived dendritic cells. Eur. J. Immunol. 29, 180–188 (1999).
Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143, 1283–1288 (1976).
Huang, A.Y. et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264, 961–965 (1994).
Kurts, C. et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184, 923–930 (1996).
Kovacsovics-Bankowski, M. & Rock, K.L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 267, 243–246 (1995).
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1, 362–368 (1999).
Huang, A.Y., Bruce, A.T., Pardoll, D.M. & Levitsky, H.I. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity 4, 349–355 (1996).
Bachmann, M.F. et al. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur. J. Immunol. 25, 1739–1743 (1995).
Schirmbeck, R., Bohm, W. & Reimann, J. Stress protein (hsp73)-mediated, TAP-independent processing of endogenous, truncated SV40 large T antigen for Db-restricted peptide presentation. Eur. J. Immunol. 27, 2016–2023 (1997).
Liu, T. et al. TAP peptide transporter-independent presentation of heat-killed Sendai virus antigen on MHC class I molecules by splenic antigen-presenting cells. J. Immunol. 159, 5364–5371 (1997).
Bakke, O. & Dobberstein, B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63, 707–716 (1990).
Jayawardena-Wolf, J., Benlagha, K., Chiu, Y.H., Mehr, R. & Bendelac, A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 15, 897–908 (2001).
Jackman, R.M. et al. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity 8, 341–351 (1998).
Pathak, S.S. & Blum, J.S. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic 1, 561–569 (2000).
Guild, B.C. & Strominger, J.L. Human and murine class I MHC antigens share conserved serine 335, the site of HLA phosphorylation in vivo. J. Biol. Chem. 259, 9235–9240 (1984).
McCluskey, J., Boyd, L.F., Maloy, W.L., Coligan, J.E. & Margulies, D.H. Alternative processing of H-2Dd pre-mRNAs results in membrane expression of differentially phosphorylated protein products. EMBO J. 5, 2477–2483 (1986).
Vega, M.A. & Strominger, J.L. Constitutive endocytosis of HLA class I antigens requires a specific portion of the intracytoplasmic tail that shares structural features with other endocytosed molecules. Proc. Natl. Acad. Sci. USA 86, 2688–2692 (1989).
Zuniga, M.C. et al. Expression and function of transplantation antigens with altered or deleted cytoplasmic domains. Cell 34, 535–544 (1983).
Murre, C. et al. Construction, expression and recognition of an H-2 molecule lacking its carboxyl terminus. Nature 307, 432–436 (1984).
Abdel Motal, U.M. et al. Glycosylphosphatidylinositol-linked Db does not induce an influenza-specific cytotoxic T lymphocyte response or recycle membrane-bound peptides. Eur. J. Immunol. 25, 1121–1124 (1995).
Rosenthal, K.L. & Zinkernagel, R.M. Inability of mice to generate cytotoxic T lymphocytes to vesicular stomatitis virus restricted to H-2Kk or H-2Dk. J. Immunol. 126, 446–451 (1981).
Imarai, M., Goyarts, E.C., van Bleek, G.M. & Nathenson, S.G. Diversity of T cell receptors specific for the VSV antigenic peptide (N52–59) bound by the H-2Kb class I molecule. Cell. Immunol. 160, 33–42 (1995).
Liu, T. et al. Heat-inactivated Sendai virus can enter multiple MHC class I processing pathways and generate cytotoxic T lymphocyte responses in vivo. J. Immunol. 154, 3147–3155 (1995).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Shastri, N. & Gonzalez, F. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J. Immunol. 150, 2724–2736 (1993).
Brossart, P. & Bevan, M.J. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood 90, 1594–1599 (1997).
Porgador, A., Yewdell, J.W., Deng, Y., Bennink, J.R. & Germain, R.N. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6, 715–726 (1997).
Norbury, C.C. et al. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J. Immunol. 166, 4355–4362 (2001).
MacAry, P.A. et al. Mobilization of MHC class I molecules from late endosomes to the cell surface following activation of CD34-derived human Langerhans cells. Proc. Natl. Acad. Sci. USA 98, 3982–3987 (2001).
Heath, W.R. & Carbone, F.R. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 1, 126–134 (2001).
Vremec, D., Pooley, J., Hochrein, H., Wu, L. & Shortman, K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164, 2978–2986 (2000).
Honing, S., Griffith, J., Geuze, H.J. & Hunziker, W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 15, 5230–5239 (1996).
Bonifacino, J.S. & Dell'Angelica, E.C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell. Biol. 145, 923–926 (1999).
Ohno, H. et al. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872–1875 (1995).
Owen, D.J. & Evans, P.R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327–1332 (1998).
Park, B. et al. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity 15, 213–224 (2001).
Ackerman, A.L. & Cresswell, P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J. Immunol. 170, 4178–4188. (2003).
Delamarre, L., Holcombe, H. & Mellman, I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J. Exp. Med. 198, 111–122 (2003).
Goldberg, A.L., Cascio, P., Saric, T. & Rock, K.L. The importance of the proteosome and subsequent proteolytic steps in the generation of antigenic peptides. Mol. Immunol. 39, 147–164 (2002).
Ramirez, M.C. & Sigal, L.J. Macrophages and dendritic cells use the cytosolic pathway to rapidly cross-present antigen from live, vaccinia-infected cells. J. Immunol. 169, 6733–6742 (2002).
Watts, C. Phagocytosis: how the phagosome became the phag-ER-some. Curr. Biol. 12, R666–668 (2002).
Desjardins, M. ER-mediated phagocytosis: a new membrane for new functions. Nat. Rev. Imm. 3, 280–291 (2003).
Gagnon, E. et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131 (2002).
Le Gall, S. et al. Nef interacts with the μ subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8, 483–495. (1998).
Andrieu, M. et al. Downregulation of major histocompatibility class I on human dendritic cells by HIV Nef impairs antigen presentation to HIV-specific CD8+ T lymphocytes. AIDS Res. Hum. Retroviruses 17, 1365–1370 (2001).
Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K. & Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 (1989).
Acknowledgements
The authors thank N. Shastri (University of California, Berkeley) for providing the B3Z T-cell hybridoma cell line, R.N. Germain (National Institutes of Health) for providing the 25.D1.16 hybridoma, J.W. Yewdell (National Institutes of Health) for providing the vaccinia viruses and conjugated 25.D1.16 antibody, and D.J. Powell, Jr. (National Institutes of Health), for critical reading of the manuscript. Supported by the Canadian Institutes of Health Research, the National Cancer Institute of Canada and the Canadian Foundation for AIDS Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Lizée, G., Basha, G., Tiong, J. et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol 4, 1065–1073 (2003). https://doi.org/10.1038/ni989
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni989
This article is cited by
-
Outside-in signaling through the major histocompatibility complex class-I cytoplasmic tail modulates glutamate receptor expression in neurons
Scientific Reports (2023)
-
The intracellular domain of major histocompatibility class-I proteins is essential for maintaining excitatory spine density and synaptic ultrastructure in the brain
Scientific Reports (2023)
-
A guide to antigen processing and presentation
Nature Reviews Immunology (2022)
-
HLA-A2.1-restricted ECM1-derived epitope LA through DC cross-activation priming CD8+ T and NK cells: a novel therapeutic tumour vaccine
Journal of Hematology & Oncology (2021)
-
Cryptic protein-protein interaction motifs in the cytoplasmic domain of MHCI proteins
BMC Immunology (2016)