Abstract
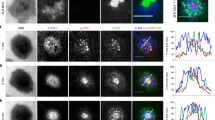
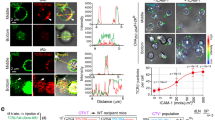
During the productive interaction of T cells with antigen-presenting cells (APCs), engaged receptors, including the T cell antigen receptors and their associated tyrosine kinases, assemble into spatially segregated supramolecular activation clusters (SMACs) at the area of cell contact. Here, we studied intracellular signaling in SMACs by three-dimensional immunofluorescence microscopic localization of CD3, CD45, talin, phosphotyrosine, Lck and phosphorylated ZAP-70 in T cell–APC conjugates. Two distinct phases of spatial-temporal activation, one before and one after SMAC formation, which were separated by a brief state of inactivation caused by CD45, were observed at the T cell–APC contact area. We propose that pre-SMAC signals are sufficient to activate cell adhesion, but not productive T cell responses, which require orchestrated signaling in SMACs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weiss, A. & Littman, D.R. Signal transduction by lymphocyte antigen receptors. Cell 76, 263–274 (1994).
Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283, 680–682 (1999).
Matsui, K. et al. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science 254, 1788–1791 (1991).
Boniface, J.J. et al. Initiation of signal transduction through the T cell receptor requires the peptide multivalent engagement of MHC ligands. Immunity 9, 459–466 (1998).
Hamad, A.R.A. et al. Potent T cell activation with dimeric peptide-major histocompatibility complex class II ligand: the role of CD4 coreceptor. J. Exp. Med. 188, 1633–1640 (1998).
Caron, L., Abraham, N., Pawson, T. & Veillette, A. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Mol. Cell. Biol. 12, 2720–2729 (1992).
Yamaguchi, H. & Hendrickson, W.A. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature 384, 484–489 (1996).
Xu, W., Harrison, S.C. & Eck, M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385, 595–602 (1997).
Sicheri, F., Moarefi, I. & Kuriyan, J. Crystal structure of the Src family tyrosine kinase Hck. Nature 385, 602–609 (1997).
Trowbridge, I.S. & Thomas, M.L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu. Rev. Immunol. 12, 85–116 (1994).
Thomas, M.L. & Brown, E.J. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol. Today 20, 406–411 (1999).
Ashwell, J.D. & D'Oro, U. CD45 and Src-family kinases: and now for something completely different. Immunol. Today 20, 412–416 (1999).
D'Oro, U. & Ashwell, J.D. The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J. Immunol. 162, 1879–1883 (1999).
Stone, J.D. et al. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ζ, and ZAP-70. J. Immunol. 158, 5773–5782 (1997).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Rodgers, W. & Rose, J.K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J. Cell Biol. 135 1515–1523 (1996).
Xavier, R., Brennan, T., Li, Q., McCormack, C. & Seed, B. Membrane compartmentation is required for efficient T cell activation. Immunity 8, 723–732 (1998).
Janes, P.W., Ley, S.C. & Magee, A.I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147, 447–461 (1999).
Monks, C.R., Freiberg, B.A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Kupfer, A. & Singer, S.J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J. Exp. Med. 170, 1697–1713 (1989).
Shenoi, H., Seavitt, J., Zheleznyak, A., Thomas, M.L. & Brown, E.J. Regulation of integrin-mediated T cell adhesion by the transmembrane protein tyrosine phosphatase CD45. J. Immunol. 162, 7120–7127 (1999).
Singer, S.J. Intercellular communication and cell-cell adhesion. Science 255, 1671–1677 (1992).
Crawford, F., Kozono, H., White, J., Marrack, P. & Kappler, J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity 8, 675–682 (1998).
Kenworthy, A.K. & Edidin, M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of 100 A using imaging fluorescence resonance energy transfer. J. Cell Biol. 142, 69–84 (1998).
Leupin, O., Zaru, R., Laroche, T., Muller, S. & Valitutti, S. Exclusion of CD45 from the T-cell receptor signaling area in antigen- stimulated T lymphocytes. Curr. Biol. 10, 277–280 (2000).
Johnson, K.G., Bromley, S.K., Dustin, M.L. & Thomas, M.L. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc. Natl. Acad. Sci. USA 97, 10138–10143 (2000).
Montixi, C. et al. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17, 5334–5348 (1998).
Leitenberg, D., Boutin, Y., Lu, D.D. & Bottomly, K. Biochemical association of CD45 with the T cell receptor complex: regulation by CD45 isoform and during T cell activation. Immunity 10, 701–711 (1999).
van Oers, N.S., Killeen, N. & Weiss, A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 183, 1053–1062 (1996).
Gold, M.R. et al. Activation and serine phosphorylation of the p56lck protein tyrosine kinase in response to antigen receptor cross-linking in B lymphocytes. J. Immunol. 153, 2369–2380 (1994).
Chan, A.C. et al. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 14, 2499–2508 (1995).
Isakov, N. et al. ZAP-70 binding specificity to T cell receptor tyrosine-based activation motifs: the tandem SH2 domains of ZAP-70 bind distinct tyrosine-based activation motifs with varying affinity. J. Exp. Med. 181, 375–380 (1995).
Mege, D. et al. Mutation of tyrosines 492/493 in the kinase domain of ZAP-70 affects multiple T-cell receptor signaling pathways. J. Biol. Chem. 271, 32644–32652 (1996).
Holdorf, A.D., Lee, K.H., Burack, W.R., Allen, P.M. & Shaw, A.S. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nature Immunol. 3, 259–264 (2002).
Lee, K.H. et al. T cell receptor signaling precedes immunological synapse formation. Science 295, 1539–1542 (2002).
Monks, C.R., Kupfer, H., Tamir, I., Barlow, A. & Kupfer, A. Selective modulation of protein kinase C-θ during T-cell activation. Nature 385, 83–86 (1997).
Acknowledgements
We thank P. Marrack, A. Weiss and G. Koretzky for helpful comments and T. Potter for critical reading of the manuscript. Supported in part by grants from the NIH (to A. K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A. K. is cofounder and co-owner of Intelligent-Imaging Innovations, Inc.
Rights and permissions
About this article
Cite this article
Freiberg, B., Kupfer, H., Maslanik, W. et al. Staging and resetting T cell activation in SMACs. Nat Immunol 3, 911–917 (2002). https://doi.org/10.1038/ni836
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni836
This article is cited by
-
A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals
Nature Immunology (2020)
-
Multi-color single-molecule tracking and subtrajectory analysis for quantification of spatiotemporal dynamics and kinetics upon T cell activation
Scientific Reports (2017)
-
In vitro tracking and intracellular protein distribution in immunology
Immunology & Cell Biology (2017)
-
Hierarchical nanostructure and synergy of multimolecular signalling complexes
Nature Communications (2016)
-
TCR-induced sumoylation of the kinase PKC-θ controls T cell synapse organization and T cell activation
Nature Immunology (2015)