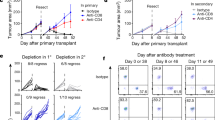
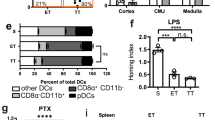
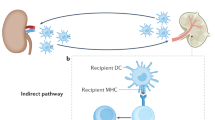
Abstract
To prevent bystander destruction of healthy host tissues, cytotoxic CD8+ T lymphocytes are fitted with specific receptors that direct their destructive forces specifically against chosen targets. We show here, however, that anti–H-Y monospecific, H-2b–restricted MataHari CD8+ T cells reject H-2k male skin grafts, with which they cannot directly interact. Such rejection is interferon-γ–dependent and only occurs if the recipient endothelium expresses H-2b. The findings suggest an alternate indirect effector pathway that requires processing and presentation of the donor H-Y antigen by recipient endothelium and have implications for both transplantation and autoimmune disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Byrne, J.A., Ahmed, R. & Oldstone, M.B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. I. Generation and recognition of virus strains and H-2b mutants. J. Immunol. 133, 433–439 (1984).
Lancki, D.W., Ma, D.I., Havran, W.L. & Fitch, F.W. Cell surface structures involved in T cell activation. Immunol. Rev. 81, 65–94 (1984).
Singer, A., Kruisbeek, A.M. & Andrysiak, P.M. T cell-accessory cell interactions that initiate allospecific cytotoxic T lymphocyte responses: existence of both Ia-restricted and Ia-unrestricted cellular interaction pathways. J. Immunol. 132, 2199–2209 (1984).
Hayry, P. & Defendi, V. Mixed lymphocyte cultures produce effector cells: model in vitro for allograft rejection. Science 168, 133–135 (1970).
Henkart, P.A. Mechanism of lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 3, 31–58 (1985).
Kagi, D. et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265, 528–530 (1994).
Ridge, J.P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 (1998).
Lassila, O., Vainio, O. & Matzinger, P. Can B cells turn on virgin T cells? Nature 334, 253–255 (1988).
Medzhitov, R. & Janeway, C.A. Jr. Innate immune recognition and control of adaptive immune responses. Semin. Immunol. 10, 351–353 (1998).
Billingham, R. & Silvers, W. Studies on tolerance of the Y chromosome antigen in mice. J. Immunol. 85, 14–26 (1960).
Desquenne-Clark, L., Chen, H.D. & Silvers, W.K. Influence of the major histocompatibility complex (MHC) on the response to and expression of H-Y in rats. Dev. Genet. 8, 189–194 (1987).
Desquenne-Clark, L., Kimura, H. & Silvers, W.K. Priming and cross-priming for H-Y in female mice. Transplantation 54, 916–919 (1992).
Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143, 1283–1288 (1976).
Matzinger, P. & Bevan, M.J. Induction of H-2-restricted cytotoxic T cells: in vivo induction has the appearance of being unrestricted. Cell. Immunol. 33, 92–100 (1977).
Arnold, D., Faath, S., Rammensee, H. & Schild, H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J. Exp. Med. 182, 885–889 (1995).
Mintz, B. & Silvers, W.K. “Intrinsic” immunological tolerance in allophenic mice. Science 158, 1484–1486 (1967).
Rosenberg, A.S. & Singer, A. Evidence that the effector mechanism of skin allograft rejection is antigen-specific. Proc. Natl. Acad. Sci. USA 85, 7739–7742 (1988).
Doody, D.P., Stenger, K.S. & Winn, H.J. Immunologically nonspecific mechanisms of tissue destruction in the rejection of skin grafts. J. Exp. Med. 179, 1645–1652 (1994).
Greenfield, A. et al. An H-YDb epitope is encoded by a novel mouse Y chromosome gene. Nature Genet. 14, 474–478 (1996).
Helms, T. et al. Direct visualization of cytokine-producing recall antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J. Immunol. 164, 3723–3732 (2000).
Matesic, D., Lehmann, P.V. & Heeger, P.S. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation 65, 906–914 (1998).
Scott, D.M. et al. Identification of a mouse male-specific transplantation antigen, H-Y. Nature 376, 695–698 (1995).
Morgan, D.J. et al. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 160, 643–651 (1998).
Sigal, L.J., Crotty, S., Andino, R. & Rock, K.L. T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 398, 77–80 (1999).
Vella, J.P. et al. Cellular and humoral mechanisms of vascularized allograft rejection induced by indirect recognition of donor MHC allopeptides. Transplantation 67, 1523–1532 (1999).
Valujskikh, A. et al. T cells reactive to a single immunodominant self-restricted allopeptide induce skin graft rejection in mice. J. Clin. Invest. 101, 1398–1407 (1998).
de Waal, R.M., Bogman, M.J., Cornelissen, I.M., Vermeulen, A.N. & Koene, R.A. Expression of donor class I major histocompatibility antigens on the vascular endothelium of mouse skin allografts. Transplantation 42, 178–183 (1986).
de Waal, R.M. et al. Variable expression of Ia antigens on the vascular endothelium of mouse skin allografts. Nature 303, 426–429 (1983).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nature Med. 6, 1348–1354 (2000).
Lapierre, L.A., Fiers, W. & Pober, J.S. Three distinct classes of regulatory cytokines control endothelial cell MHC antigen expression. Interactions with immune γ interferon differentiate the effects of tumor necrosis factor and lymphotoxin from those of leukocyte α and fibroblast β interferons. J. Exp. Med. 167, 794–804 (1988).
Pober, J.S. & Gimbrone, M.A. Jr. Expression of Ia-like antigens by human vascular endothelial cells is inducible in vitro: demonstration by monoclonal antibody binding and immunoprecipitation. Proc. Natl. Acad. Sci. USA 79, 6641–6645 (1982).
Hasegawa, S., Becker, G., Nagano, H., Libby, P. & Mitchell, R.N. Pattern of graft- and host-specific MHC class II expression in long- term murine cardiac allografts: origin of inflammatory and vascular wall cells. Am. J. Pathol. 153, 69–79 (1998).
Kreisel, D. et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nature Med. 8, 233–239 (2002).
Jones, N.D. et al. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J. Immunol. 166, 2824–2830 (2001).
Colucci, F. et al. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK cell differentiation. J. Immunol. 162, 2761–2765 (1999).
Lantz, O., Grandjean, I., Matzinger, P. & Di Santo, J.P. γ chain required for naive CD4+ T cell survival but not for antigen proliferation. Nature Immunol. 1, 54–58 (2000).
Bevan, M.J. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 117, 2233–2238 (1976).
Epperson, D.E. et al. Cytokines increase transporter in antigen processing-1 expression more rapidly than HLA class I expression in endothelial cells. J. Immunol. 149, 3297–3301 (1992).
Ma, W., Lehner, P.J., Cresswell, P., Pober, J.S. & Johnson, D.R. Interferon-γ rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. J. Biol. Chem. 272, 16585–16590 (1997).
Miura, M. et al. Monokine induced by IFN-γ is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J. Immunol. 167, 3494–3504 (2001).
Silvers, W.K., Billingham, R.E. & Sanford, B.H. The H-Y transplantation antigen: a Y-linked or sex-influenced factor? Nature 220, 401–403 (1968).
Lee, R.S. et al. CD8+ effector cells responding to residual class I antigens, with help from CD4+ cells stimulated indirectly, cause rejection of “major histocompatibility complex-deficient” skin grafts. Transplantation 63, 1123–1133 (1997).
Quaini, F. et al. Chimerism of the transplanted heart. N. Engl. J. Med. 346, 5–15 (2002).
Huseby, E.S. et al. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J. Exp. Med. 194, 669–676 (2001).
Marincola, F.M., Jaffee, E.M., Hicklin, D.J. & Ferrone, S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273 (2000).
Marincola, F.M. et al. Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J. Immunol. 153, 1225–1237 (1994).
Jakobsen, M.K. et al. Defective major histocompatibility complex class I expression in a sarcomatoid renal cell carcinoma cell line. J. Immunother. Emphasis Tumor Immunol. 17, 222–228 (1995).
Plautz, G.E., Mukai, S., Cohen, P.A. & Shu, S. Cross-presentation of tumor antigens to effector T cells is sufficient to mediate effective immunotherapy of established intracranial tumors. J. Immunol. 165, 3656–3662 (2000).
Wigginton, J.M. et al. IFN-γ and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J. Clin. Invest. 108, 51–62 (2001).
Patten, P.A. et al. Transfer of putative complementarity-determining region loops of T cell receptor V domains confers toxin reactivity but not peptide/MHC specificity. J. Immunol. 150, 2281–2294 (1993).
Kreisel, D. et al. A simple method for culturing mouse vascular endothelium. J. Immunol. Meth. 254, 31–45 (2001).
Heeger, P.S., Valujskikh, A. & Lehmann, P.V. Comprehensive assessment of determinant specificity, frequency, and cytokine signature of the primed CD8 cell repertoire induced by a minor transplantation antigen. J. Immunol. 165, 1278–1284 (2000).
Matesic, D., Lehmann, P.V. & Heeger, P.S. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation 65, 906–914 (1998).
Matzinger, P. The JAM test. A simple assay for DNA fragmentation and cell death. J. Immunol. Meth. 145, 185–192 (1991).
Hancock, W.W., Buelow, R., Sayegh, M.H. & Turka, L.A. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nature Med. 4, 1392–1396 (1998).
Chan, S.Y., DeBruyne, L.A., Goodman, R.E., Eichwald, E.J. & Bishop, D.K. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation 59, 1155–1161 (1995).
Acknowledgements
Funded by National Institutes of Health grant AI-43578-01 (to P. S. H.); grants from the Etablissement Français des greffes, Association de la recherche sur le cancer and the French ministry of Research (to O. L.); and the National Kidney Foundation (P. S. H.). We thank X. Sastre for analyzing the skin graft histology; Y. Demir for help with immunohistochemical staining; and E. Skornicka for help with isolation of the endothelial cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Web Fig. 1.
Skin graft histology. H&E-stained B6 male (a,b) or C3H male (c-e) skin grafts obtained on day 7-15 after placement on female MataHari recipients. Note that the B6 grafts are extensively infiltrated with mononuclear cells and there is evidence of epidermal necrosis. The C3H grafts are characterized by marked fibrosis with little necrosis. The photographs are fully representative of 5 individual male C3H grafts and 3 individual male B6 grafts studied. Magnification: a-d: 40×; e: 100×. Histologic characteristics of isografts and C3H female grafts were no different from native skin and revealed no evidence of infiltration or fibrosis (data not shown). (PDF 305 kb)
Web Fig. 2.
Placement of C3H male heart grafts cross primes MataHari T cells in vivo. (a) Flow cytometry histogram using spleen cells obtained from a naive MataHari female (top) or a MataHari female 70 days after placement of an H-2k male heart transplant (bottom) after gating on Vβ8.3+ T cells. (solid line) anti-CD44, (dashed line) isotype control. The results are representative of 3 individual animals studied per group. (b) IFN-γ ELISPOT assays using spleen cells from naïve MataHari females or MataHari females 70 days after transplantation with a C3H male heart graft mice restimulated with H-Ypb. Each line represents the results of an individual animal. Each point is the mean value of duplicate wells as counted by our computer assisted image analyzer (<10% variability between wells). (PDF 37 kb)
Web Fig. 3.
Histology of skin grafts in RAG-1-/-γc-/- recipient mice. H&E-stained sections of skin grafts obtained 6 weeks after transplantation. H-2k male skin was placed either onto H-2k RAG-1-/-γc-/- female recipients (a) or H-2b RAG-1-/-γc-/- female recipients (b,c) adoptively transferred with female MataHari spleen cells. Note the lack of hair follicles and sebaceous glands (b) and focal mononuclear cell infiltrates c. The photographs are fully representative of the histologic findings for 3-6 animals per group. (PDF 191 kb)
Rights and permissions
About this article
Cite this article
Valujskikh, A., Lantz, O., Celli, S. et al. Cross-primed CD8+ T cells mediate graft rejection via a distinct effector pathway. Nat Immunol 3, 844–851 (2002). https://doi.org/10.1038/ni831
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni831
This article is cited by
-
The glucose transporter 2 regulates CD8+ T cell function via environment sensing
Nature Metabolism (2023)
-
Immune reaction and regulation in transplantation based on pluripotent stem cell technology
Inflammation and Regeneration (2020)
-
Preservation of microvascular barrier function requires CD31 receptor-induced metabolic reprogramming
Nature Communications (2020)
-
The importance of non-HLA antibodies in transplantation
Nature Reviews Nephrology (2016)
-
The CD8 T‐cell response during tolerance induction in liver transplantation
Clinical & Translational Immunology (2016)