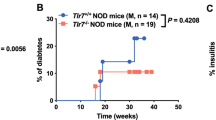
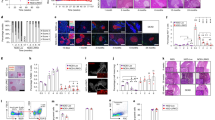
Abstract
The mechanisms that regulate susceptibility to virus-induced autoimmunity remain undefined. We establish here a fundamental link between the responsiveness of target pancreatic β cells to interferons (IFNs) and prevention of coxsackievirus B4 (CVB4)-induced diabetes. We found that an intact β cell response to IFNs was critical in preventing disease in infected hosts. The antiviral defense, raised by β cells in response to IFNs, resulted in a reduced permissiveness to infection and subsequent natural killer (NK) cell–dependent death. These results show that β cell defenses are critical for β cell survival during CVB4 infection and suggest an important role for IFNs in preserving NK cell tolerance to β cells during viral infection. Thus, alterations in target cell defenses can critically influence susceptibility to disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Hyoty, H., Hiltunen, M. & Lonnrot, M. Enterovirus infections and insulin dependent diabetes mellitus – evidence for causality. Clin. Diagn. Virol. 9, 77–84 (1998).
Knip, M. & Akerblom, H. K. Environmental factors in the pathogenesis of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 107, 93–100 (1999).
Robles, D. T. & Eisenbarth, G. S. Type 1a diabetes induced by infection and immunization. J. Autoimmun. 16, 355–362 (2001).
Horwitz, M. S. & Sarvetnick, N. Viruses, host responses, and autoimmunity. Immunol. Rev. 169, 241–253 (1999).
Whitton, J. L. & Fujinami, R. S. Viruses as triggers of autoimmunity: facts and fantasies. Curr. Opin. Microbiol. 2, 392–397 (1999).
Kukreja, A. & Maclaren, N. K. Current cases in which epitope mimicry is considered as a component cause of autoimmune disease: immune-mediated (type 1) diabetes. Cell. Mol. Life Sci. 57, 534–541 (2000).
Gladisch, R., Hofmann, W. & Waldherr, R. Myocarditis and insulitis following coxsackie virus infection. Z. Kardiol. 65, 837–849 (1976).
Jenson, A. B., Rosenberg, H. S. & Notkins, A. L. Pancreatic islet-cell damage in children with fatal viral infections. Lancet 2, 354–358 (1980).
Foulis, A. K., McGill, M., Farquharson, M. A. & Hilton, D. A. A search for evidence of viral infection in pancreases of newly diagnosed patients with IDDM. Diabetologia 40, 53–61 (1997).
Yoon, J. W., Austin, M., Onodera, T. & Notkins, A. L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300, 1173–1179 (1979).
Szopa, T. M., Gamble, D. R. & Taylor, K. W. Coxsackie B4 virus induces short-term changes in the metabolic functions of mouse pancreatic islets in vitro. Cell. Biochem. Funct. 4, 181–187 (1986).
Szopa, T. M., Dronfield, D. M., Ward, T. & Taylor, K. W. In vivo infection of mice with Coxsackie B4 virus induces long-term functional changes in pancreatic islets with minimal alteration in blood glucose. Diabet. Med. 6, 314–319 (1989).
Frisk, G., Grapengiesser, E. & Diderholm, H. Impaired Ca2+ response to glucose in mouse β-cells infected with coxsackie B or Echo virus. Virus Res. 33, 229–240 (1994).
Roivainen, M. et al. Mechanisms of coxsackievirus-induced damage to human pancreatic β-cells. J. Clin. Endocrinol. Metab. 85, 432–440 (2000).
Vreugdenhil, G. R. et al. Acute onset of type I diabetes mellitus after severe echovirus 9 infection: putative pathogenic pathways. Clin. Infect. Dis. 31, 1025–1031 (2000).
Ramsingh, A. I., Chapman, N. & Tracy, S. Coxsackieviruses and diabetes. Bioessays 19, 793–800 (1997).
Horwitz, M. S. et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nature Med. 4, 781–785 (1998).
Mena, I. et al. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology 271, 276–288 (2000).
Flodström, M. et al. A critical role for inducible nitric oxide synthase (NOS2) in host survival following Coxsackievirus B4 infection. Virology 281, 205–215 (2001).
Torres, A., Garib, J. & Recurt, M. L. Coxsackie virus: a review. Bol. Asoc. Med. P. R. 76, 49–51 (1984).
Ramsingh, A. I., Lee, W. T., Collins, D. N. & Armstrong, L. E. T cells contribute to disease severity during coxsackievirus B4 infection. J. Virol. 73, 3080–3086 (1999).
Nigro, G., Pacella, M. E., Patane, E. & Midulla, M. Multi-system coxsackievirus B-6 infection with findings suggestive of diabetes mellitus. Eur. J. Pediatr. 145, 557–559 (1986).
Champsaur, H. et al. Diabetes and Coxsackie virus B5 infection. Lancet 1, 251 (1980).
Yoon, J. W., McClintock, P. R., Onodera, T. & Notkins, A. L. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J. Exp. Med. 152, 878–892 (1980).
Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Goodbourn, S., Didcock, L. & Randall, R. E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81, 2341–2364 (2000).
Le Page, C., Genin, P., Baines, M. G. & Hiscott, J. Interferon activation and innate immunity. Rev. Immunogenet. 2, 374–386 (2000).
Ihle, J. N. The Stat family in cytokine signaling. Curr. Opin. Cell. Biol. 13, 211–217 (2001).
Heitmeier, M. R., Scarim, A. L. & Corbett, J. A. Prolonged STAT1 activation is associated with interferon-γ priming for interleukin-1-induced inducible nitric-oxide synthase expression by islets of Langerhans. J. Biol. Chem. 274, 29266–29273 (1999).
Hamaguchi, K., Gaskins, H. R. & Leiter, E. H. NIT-1, a pancreatic β-cell line established from a transgenic NOD/Lt mouse. Diabetes 40, 842–849 (1991).
Sakamoto, H., Kinjyo, I. & Yoshimura, A. The janus kinase inhibitor, Jab/SOCS-1, is an interferon-γ inducible gene and determines the sensitivity to interferons. Leuk. Lymphoma 38, 49–58 (2000).
Kile, B. T., Nicola, N. A. & Alexander, W. S. Negative regulators of cytokine signaling. Int. J. Hematol. 73, 292–298 (2001).
Sarvetnick, N., Liggitt, D., Pitts, S. L., Hansen, S. E. & Stewart, T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-γ. Cell 52, 773–782 (1988).
Cottet, S. et al. SOCS-1 protein prevents JAK/STAT-dependent inhibition of β cell insulin gene transcription and secretion in response to IFN-γ. J. Biol. Chem. 276, 25862–25870 (2001).
Frisk, G. & Diderholm, H. Tissue culture of isolated human pancreatic islets infected with different strains of coxsackievirus B4: assessment of virus replication and effects on islet morphology and insulin release. Int. J. Exp. Diabetes Res. 1, 165–175 (2000).
van den Broek, M. F., Muller, U., Huang, S., Zinkernagel, R. M. & Aguet, M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148, 5–18 (1995).
Kerr, J. F., Gobe, G. C., Winterford, C. M. & Harmon, B. V. Anatomical methods in cell death. Meth. Cell. Biol. 46, 1–27 (1995).
Serreze, D. V., Ottendorfer, E. W., Ellis, T. M., Gauntt, C. J. & Atkinson, M. A. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 49, 708–711 (2000).
Wicker, L. S., Todd, J. A. & Peterson, L. B. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 13, 179–200 (1995).
Horwitz, M. S., Krahl, T., Fine, C., Lee, J. & Sarvetnick, N. Protection from lethal coxsackievirus-induced pancreatitis by expression of γ interferon. J. Virol. 73, 1756–1766 (1999).
Rueckert, R. in Fields Virology (eds. Knipe, D. M. et al.) 609–654 (Lippincott - Raven Publishers, Philadelphia, PA, 1996).
Abbas, A. K., Lichtman, A. H. & Pober, J. S. Cellular and Molecular Immunology (W. B. Saunders Company, Philadelphia, PA, 2000).
Bach, J. F. & Mathis, D. The NOD mouse. Res. Immunol. 148, 285–286 (1997).
Prochazka, M., Gaskins, H. R., Shultz, L. D. & Leiter, E. H. The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc. Natl Acad. Sci. USA 89, 3290–3294 (1992).
Tay, C. H., Szomolanyi-Tsuda, E. & Welsh, R. M. Control of infections by NK cells. Curr. Top. Microbiol. Immunol. 230, 193–220 (1998).
Biron, C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17, 189–220 (1999).
Wiltrout, R. H., Salup, R. R., Twilley, T. A. & Talmadge, J. E. Immunomodulation of natural killer activity by polyribonucleotides. J. Biol. Response Mod. 4, 512–517 (1985).
Kasai, M. et al. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature 291, 334–335 (1981).
Lee, U., Santa, K., Habu, S. & Nishimura, T. Murine asialo GM1+CD8+ T cells as novel interleukin-12-responsive killer T cell precursors. Jpn. J. Cancer Res. 87, 429–432 (1996).
Doherty, P. C. & Allan, J. E. Anti-asialo GM1 eliminates both inflammatory process and cytotoxic T-cell function in the lymphocytic choriomeningitis adoptive transfer model. Cell. Immunol. 107, 1–7 (1987).
Parker, S. E., Sun, Y. H. & Sears, D. W. Differential expression of the ASGM1 antigen on anti-reovirus and alloreactive cytotoxic T lymphocytes (CTL). J. Immunogenet. 15, 215–226 (1988).
Slifka, M. K., Pagarigan, R. R. & Whitton, J. L. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 164, 2009–2015 (2000).
Gombert, J. M. et al. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur. J. Immunol. 26, 2989–2998 (1996).
Harada, M. et al. Natural killer cells inhibit the development of autoantibody production in (C57BL/6 x DBA/2) F1 hybrid mice injected with DBA/2 spleen cells. Cell. Immunol. 161, 42–49 (1995).
Godeny, E. K. & Gauntt, C. J. Murine natural killer cells limit coxsackievirus B3 replication. J. Immunol. 139, 913–918 (1987).
Vella, C. & Festenstein, H. Coxsackievirus B4 infection of the mouse pancreas: the role of natural killer cells in the control of virus replication and resistance to infection. J. Gen. Virol. 73, 1379–1386 (1992).
Bergelson, J. M. et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323 (1997).
Bergelson, J. M. et al. Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J. Infect. Dis. 175, 697–700 (1997).
Frisk, G., Elfstrom, T. & Diderholm, H. The replication of certain Coxsackie B virus strains in CHO cells. J. Virol. Meth. 98, 161–165 (2001).
Chehadeh, W. et al. Persistent infection of human pancreatic islets by coxsackievirus B is associated with α interferon synthesis in β cells. J. Virol. 74, 10153–10164 (2000).
Tomko, R. P., Xu, R. & Philipson, L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl Acad. Sci. USA 94, 3352–3356 (1997).
Bergelson, J. M. et al. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72, 415–419 (1998).
Lyles, D. S. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64, 709–724 (2000).
Foulis, A. K., Farquharson, M. A. & Meager, A. Immunoreactive α-interferon in insulin-secreting β cells in type 1 diabetes mellitus. Lancet 2, 1423–1427 (1987).
Somoza, N. et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V β usage, and cytokine profile. J. Immunol. 153, 1360–1377 (1994).
Huang, X. et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes 44, 658–664 (1995).
Rhodes, C. J. & Taylor, K. W. Effect of human lymphoblastoid interferon on insulin synthesis and secretion in isolated human pancreatic islets. Diabetologia 27, 601–603 (1984).
Rhodes, C. J. & Taylor, K. W. Effect of interferon and double-stranded RNA on B-cell function in mouse islets of Langerhans. Biochem. J. 228, 87–94 (1985).
Shimizu, F., Shimizu, M. & Kamiyama, K. Inhibitory effect of interferon on the production of insulin. Endocrinology 117, 2081–2084 (1985).
Stewart, T. A. et al. Induction of type I diabetes by interferon-α in transgenic mice. Science 260, 1942–1946 (1993).
Pelegrin, M. et al. Evidence from transgenic mice that interferon-β may be involved in the onset of diabetes mellitus. J. Biol. Chem. 273, 12332–12340 (1998).
Stauffer, Y. et al. Interferon-α-induced endogenous superantigen. a model linking environment and autoimmunity. Immunity 15, 591–601 (2001).
Imagawa, A., Hanafusa, T., Miyagawa, J. & Matsuzawa, Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N. Engl. J. Med. 342, 301–307 (2000).
Imagawa, A., Hanafusa, T., Miyagawa, J. & Matsuzawa, Y. A proposal of three distinct subtypes of type 1 diabetes mellitus based on clinical and pathological evidence. Ann. Med. 32, 539–543 (2000).
Bach, J. F. New concepts of the etiopathogenesis and treatment of insulin-dependent diabetes mellitus. Clin. Rev. Allergy Immunol. 19, 217–225 (2000).
Hiltunen, M. et al. Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. Childhood Diabetes in Finland (DiMe) Study Group. J. Infect. Dis. 175, 554–560 (1997).
Andreoletti, L. et al. Coxsackie B virus infection and β cell autoantibodies in newly diagnosed IDDM adult patients. Clin. Diagn. Virol. 9, 125–133 (1998).
Lönnrot, M. et al. Enterovirus infection as a risk factor for β-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes 49, 1314–1318 (2000).
Luppi, P. et al. Restricted TCR V β gene expression and enterovirus infection in type I diabetes: a pilot study. Diabetologia 43, 1484–1497 (2000).
Ludewig, B., Junt, T., Hengartner, H. & Zinkernagel, R. M. Dendritic cells in autoimmune diseases. Curr. Opin. Immunol. 13, 657–662 (2001).
Graves, P. M., Norris, J. M., Pallansch, M. A., Gerling, I. C. & Rewers, M. The role of enteroviral infections in the development of IDDM: limitations of current approaches. Diabetes 46, 161–168 (1997).
Endo, T. A. et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387, 921–924 (1997).
Naka, T. et al. Structure and function of a new STAT-induced STAT inhibitor. Nature 387, 924–929 (1997).
Starr, R. et al. A family of cytokine-inducible inhibitors of signalling. Nature 387, 917–921 (1997).
Flodström, M. & Eizirik, D. L. Interferon-γ-induced interferon regulatory factor-1 (IRF-1) expression in rodent and human islet cells precedes nitric oxide production. Endocrinology 138, 2747–2753 (1997).
Flodström, M., Tyrberg, B., Eizirik, D. L. & Sandler, S. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes 48, 706–713 (1999).
Acknowledgements
We thank L. Mocknic, A. Ilic and L. Tucker for excellent technical assistance; N. Hill, M. Horwitz, C. King, M. Kritzik, S. Pakala, F. Shi and other members of the Sarvetnick laboratory for discussions and suggestions; P. Minick for editing the manuscript; and B. Smith and M. Wood (Core Electron Microscope Facility, The Scripps Research Institute) for assistance with electron and confocal microscopy. Supported by National Institutes of Health grants (ROI: AI42231), the National Multiple Sclerosis Society (M. F.) and the Juvenile Diabetes Research Foundation (M. F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Flodström, M., Maday, A., Balakrishna, D. et al. Target cell defense prevents the development of diabetes after viral infection. Nat Immunol 3, 373–382 (2002). https://doi.org/10.1038/ni771
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni771
This article is cited by
-
Covid 19 and diabetes in children: advances and strategies
Diabetology & Metabolic Syndrome (2024)
-
Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus
Nature Reviews Endocrinology (2022)
-
Pancreatic beta cells persistently infected with coxsackievirus B4 are targets of NK cell-mediated cytolytic activity
Cellular and Molecular Life Sciences (2020)
-
Impact of coxsackievirus-B4E2 combined with a single low dose of streptozotocin on pancreas of outbred mice: investigation of viral load, pathology and inflammation
Scientific Reports (2019)
-
A viral link for type 1 diabetes
Nature Medicine (2019)