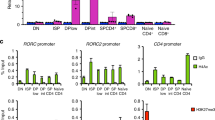
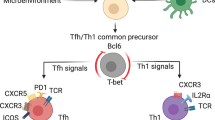
Abstract
The NFAT family of transcription factors are key regulators of inducible gene expression in the immune system. We examined the function of two NFAT proteins after naïve T helper (TH) cell activation. We found that naïve TH precursors that are doubly deficient in NFATc2 and NFATc3 intrinsically differentiate into TH2-secreting cells, even in the absence of interleukin 4 (IL-4) production. We also found that lack of NFATc2 and NFATc3 obviates the necessity for engagement of CD28 on naïve cells and controls the time required to reach the first cell division upon activation. These results demonstrate a key role for NFATc2 and NFATc3 in modulating T cell receptor responsiveness and regulating subsequent cell division and TH2 differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lanzavecchia, A., Iezzi, G. & Viola, A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell 96, 1–4 (1999).
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Rengarajan, J., Szabo, S. J. & Glimcher, L. H. Transcriptional regulation of Th1/Th2 polarization. Immunol. Today 21, 479–483 (2000).
Rao, A., Luo, C. & Hogan, P. G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Rengarajan, J. et al. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity 12, 293–300 (2000).
Ranger, A. M., Oukka, M., Rengarajan, J. & Glimcher, L. H. Inhibitory function of two NFAT family members in lymphoid homeostasis and Th2 development. Immunity 9, 627–635 (1998).
Szabo, S. J., Glimcher, L. H. & Ho, I. C. Genes that regulate interleukin-4 expression in T cells. Curr. Opin. Immunol. 9, 776–781 (1997).
Agarwal, S., Avni, O. & Rao, A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity 12, 643–652 (2000).
Ranger, A. M. et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NFATc. Immunity 8, 125–134 (1998).
Peng, S. L., Gerth, A. J., Ranger, A. M. & Glimcher, L. H. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 14, 13–20 (2001).
Hodge, M. R. et al. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp- deficient mice. Immunity 4, 397–405 (1996).
Xanthoudakis, S. et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science 272, 892–895 (1996).
Kiani, A., Rao, A. & Aramburu, J. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity 12, 359–372 (2000).
Oukka, M. et al. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity 9, 295–304 (1998).
Seder, R. A. & Paul, W. E. in Annu. Rev. Immunol (eds. Paul, W. E. et al.) 635–673 (Annual Reviews, Palo Alto, CA, 1994).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naïve and effector T cells. Immunity 8, 89–95 (1998).
Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283, 680–682 (1999).
Gett, A. V. & Hodgkin, P. D. A cellular calculus for signal integration by T cells. Nature Immunol. 1, 239–244 (2000).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Wulfing, C. & Davis, M. M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Gett, A. V. & Hodgkin, P. D. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc. Natl. Acad. Sci. USA 95, 9488–9493 (1998).
Bird, J. J. et al. Helper T cell differentiation is controlled by the cell cycle. Immunity 9, 229–237 (1998).
Richter, A., Lohning, M. & Radbruch, A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J. Exp. Med. 190, 1439–1450 (1999).
Kubo, M. et al. CD28 costimulation accelerates IL-4 receptor sensitivity and IL-4- mediated Th2 differentiation. J. Immunol. 163, 2432–2442 (1999).
Oosterwegel, M. A. et al. The role of CTLA-4 in regulating Th2 differentiation. J. Immunol. 163, 2634–2639 (1999).
Rulifson, I. C., Sperling, A. I., Fields, P. E., Fitch, F. W. & Bluestone, J. A. CD28 costimulation promotes the production of Th2 cytokines. J. Immunol. 158, 658–665 (1997).
Kuhn, R., Rajewsky, K. & Muller, W. Generation and analysis of interleukin-4 deficient mice. Science 254, 707–710 (1991).
McKenzie, G. J. et al. Impaired development of Th2 cells in IL-13–deficient mice. Immunity 9, 423–432 (1998).
McKenzie, G. J., Fallon, P. G., Emson, C. L., Grencis, R. K. & McKenzie, A. N. J. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 189, 1565–1572 (1999).
Ouyang, W. et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity 12, 27–37 (2000).
Ranganath, S. et al. GATA-3-dependent enhancer activity in IL-4 gene regulation. J. Immunol. 161, 3822–3826 (1998).
Ouyang, W. et al. Inhibition of Th1 developmental mediated by GATA-3 through an IL-4 independent mechanism. Immunity 9, 745–755 (1998).
Acknowledgements
We thank A. Wurster, S. Szabo and other members of the laboratory for discussions and comments on the manuscript; M. Handly and R. McGilp for FACS expertise; and C. McCall for preparing the manuscript. Supported by the National Institutes of Health AI31541-05 (L. H. G.), the G. Harold and Leila Y. Mathers Charitable Foundation (L. H. G.) and Fogarty International (J. R.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rengarajan, J., Tang, B. & Glimcher, L. NFATc2 and NFATc3 regulate TH2 differentiation and modulate TCR-responsiveness of naïve TH cells. Nat Immunol 3, 48–54 (2002). https://doi.org/10.1038/ni744
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni744
This article is cited by
-
High miR203a-3p and miR-375 expression in the airways of smokers with and without COPD
Scientific Reports (2022)
-
CML derived exosomes promote tumor favorable functional performance in T cells
BMC Cancer (2021)
-
NFAT2 overexpression suppresses the malignancy of hepatocellular carcinoma through inducing Egr2 expression
BMC Cancer (2020)
-
Targeted deletion of NFAT-Interacting-Protein-(NIP) 45 resolves experimental asthma by inhibiting Innate Lymphoid Cells group 2 (ILC2)
Scientific Reports (2019)
-
Immunomodulatory and therapeutic role of Cinnamomum verum extracts in collagen-induced arthritic BALB/c mice
Inflammopharmacology (2018)