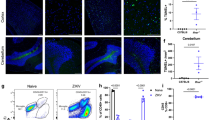
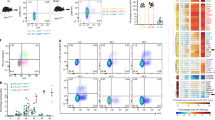
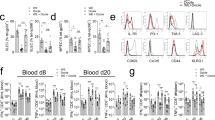
Abstract
Cytomegalovirus (CMV), measles and HIV are the main human pathogens known to induce immunosuppression. Unlike measles and HIV, and despite the availability of a well studied animal model, little is known about the mechanisms that control CMV-induced immunosuppression. We hypothesized that dendritic cells (DCs), which are crucial in generating and maintaining immune responses, represent a target for CMV and that the transient, but profound, immunosuppression that accompanies CMV infection results from viral interference with DC functions. Here we show that DCs were permissive to murine CMV infection. In addition, DC infection prevented delivery of the signals required for T cell activation. Thus, CMV-mediated impairment of DC function may be crucial for virally induced immunosuppression and interleukin 2 is implicated as a key factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Naniche, D. & Oldstone, M. B. A. Generalized immunosuppression: how viruses undermine the immune response. Cell. Mol. Life. Sci. 57, 1399–1407 (1999).
Alford, C. A. & Britt, W. J. in Virology (ed. Fields, B. N.) 2493–2523 (Raven Press, New York, 1996).
Koszinowski, U. H., Jonjic, S. & Lucin, P. Cytomegalovirus persistence by evasion from immune control. Semin. Virol. 5, 297–312 (1994).
Hengel, H., Brune, W. & Koszinowski, U. H. Immune evasion by cytomegalovirus–survival strategies of a highly adapted opportunist. Trends Microbiol. 6, 190–197 (1998).
Hengel, H. et al. Cytomegaloviral control of MHC class I function in the mouse. Immunol. Rev. 168, 167–176 (1999).
Heise, M. T. et al. Murine cytomegalovirus infection inhibits IFN γ-induced MHC class II expression on macrophages: the role of type I interferon. Virology 241, 331–344 (1998).
Redpath, S., Angulo, A., Gascoigne, N. R. J. & Ghazal, P. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162, 6701–6707 (1999).
Fleming, P. et al. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J. Virol. 73, 6800–6809 (1999).
Farrell, H. E. et al. Inhibition of natural killer cells by a cytomegalovirus MHC class I homologue in vivo. Nature 386, 510–514 (1997).
Heise, M. T., Connick, M. & Virgin, H. W. t. Murine cytomegalovirus inhibits interferon γ-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J. Exp. Med. 187, 1037–1046 (1998).
Farrell, H. E., Degli-Esposti, M. A. & Davis-Poynter, N. J. Cytomegalovirus evasion of natural killer cell responses. Immunol. Rev. 168, 187–197 (1999).
Stoddart, C. A. et al. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68, 6243–6253 (1994).
Collins, T. M., Quirk, M. R. & Jordan, M. C. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J. Virol. 68, 6305–6311 (1994).
Hanson, L. K. et al. Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis. J. Virol. 73, 5970–5980 (1999).
Riegler, S. et al. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81, 393–399 (2000).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Austyn, J. M. New insights into the mobilization and phagocytic activity of dendritic cells. J. Exp. Med. 183, 1287–1292 (1996).
De Smedt, T. et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184, 1413–1424 (1996).
Rescigno, M., Granucci, F. & Ricciardi-Castagnoli, P. Dendritic cells at the end of the Millennium. Immunol. Cell Biol. 77, 404–410 (1999).
Winzler, C. et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185, 317–28 (1997).
Granucci, F. et al. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1, 1079–1084 (1999).
Schuurhuis, D. H. et al. Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J. Exp. Med. 192, 145–150 (2000).
Suri, R. M. & Austyn, J. M. Bacterial lipopolysaccharide contamination of commercial collagen preparations may mediate dendritic cell maturation in culture. J. Immunol. Meth. 214, 149–163 (1998).
Vremec, D., Pooley, J., Hochrein, H., Wu, L. & Shortman, K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164, 2978–2986 (2000).
Davis-Poynter, N. J. et al. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J. Virol. 71, 1521–1529 (1997).
Granucci, F. et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nature Immunol. 2, 882–888 (2001).
Rinaldo, C. R. Immune suppression by herpesviruses. Ann. Rev. Med. 41, 331–338 (1990).
Hirsch, M. S. & Felsenstein, D. CMV-associated immunosuppression. Ann. NY Acad. Sci. 437, 8–15 (1984).
Rubin, R. H. in Clinical Approach to Infections in the Immunocompromised Patient (eds. Rubin, R. H. & Young, L. S.) 557–583 (Plenum Press, New York, 1988).
Kay, M. A., Glorioso, J. C. & Naldini, L. Viral vectors for gene therapy: the art of turning infectious agents into vehicle therapeutics. Nature Med. 7, 33–40 (2001).
Garrett, W. S. et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102, 325–334 (2000).
Sallusto, F., Cella, M., Danieli, C. & Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment - downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 (1995).
Hengel, H. et al. Macrophages escape inhibition of major histocompatibility complex class I-dependent antigen presentation by cytomegalovirus. J. Virol. 74, 7861–7868 (2000).
Turley, S. J. et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science 288, 522–527 (2000).
Karre, K. How to recognize a foreign submarine. Immunol. Rev. 155, 5–9 (1997).
Chambers, B. J., Salcedo, M. & Ljunggren, H. G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1). Immunity 5, 311–317 (1996).
Cretney, E. et al. m144, a murine cytomegalovirus (MCMV)-encoded major histocompatibility complex class I homologue, confers tumor resistance to natural killer cell-mediated rejection. J. Exp. Med. 190, 435–444 (1999).
Langenkamp, A., Messi, M., Lanzavecchia, A. & Sallusto, F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and non-polarized T cells. Nature Immunol. 1, 311–316 (2000).
Waldmann, T. A., Dubois, S. & Tagaya, Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 14, 105–110 (2001).
Timon, M. et al. Selective impairment of T lymphocyte activation through the T cell receptor/CD3 complex after cytomegalovirus infection. Clin. Exp. Immunol. 94, 38–42 (1993).
Kapasi, K. & Rice, G. P. A. Cytomegalovirus infection of peripheral blood mononuclear cells: effects on interleukin-1 and -2 production and responsiveness. J. Virol. 62, 3603–3607 (1988).
Blackett, S. & Mims, C. A. Studies of depressed interleukin-2 production by spleen cells from mice following infection with cytomegalovirus. Arch. Virol. 99, 1–8 (1988).
Reddehase, M. J., Mutter, W. & Koszinowski, U. H. In vivo application of recombinant interleukin-2 in the immunotherapy of established cytomegalovirus infection. J. Exp. Med. 165, 650–656 (1987).
Slater, J. S., Futch, W. S., Cavanaugh, V. J. & Campbell, A. E. Murine cytomegalovirus independently inhibits priming of helper and cytotoxic T lymphocytes. Virology 185, 132–139 (1991).
Carney, W. P. & Hirsch, M. S. Mechanisms of immunosuppression in cytomegalovirus mononucleosis. II. Virus-monocyte interactions. J. Infect. Dis. 144, 47–54 (1981).
Kapasi, K. & Rice G. P. A. Role of the monocyte in cytomegalovirus-mediated immunosuppression in vitro. J. Infect. Dis. 154, 881–884 (1986).
Yoshida, H. et al. Induction of apoptosis of T cells by infecting mice with murine cytomegalovirus. J. Virol. 69, 4769–4775 (1995).
Vremec, D. et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 176, 47–58 (1992).
Maraskovsky, E. et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184, 1953–1962 (1996).
Hochrein, H. et al. Interleukin(IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J. Exp. Med. 192, 823–833 (2000).
Acknowledgements
We thank M. Wikstrom (The Lotteries Commission of Western Australia Flow Cytometry and Cell Sorting Facility) for assistance with the purification of DCs by cell sorting; H. Tabarias for help with the cytokine ELISA assays; and E. Maraskovsky, M. Smyth, G. Shellam, A Strasser, G. Begley, H. Farrell and A. Scalzo for continued support, critical discussions and reading of this manuscript. Supported by the National Health and Medical Research Council of Australia (grants 110287, 110288), a Wellcome Trust Overseas Senior Research Fellowship in Biomedical Science in Australia (M. A. D.-E.) and AMRAD (D. M. A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrews, D., Andoniou, C., Granucci, F. et al. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol 2, 1077–1084 (2001). https://doi.org/10.1038/ni724
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni724
This article is cited by
-
Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection
Medical Microbiology and Immunology (2019)
-
Cytomegalovirus immune evasion of myeloid lineage cells
Medical Microbiology and Immunology (2015)
-
Priming of T follicular helper cells by dendritic cells
Immunology & Cell Biology (2014)
-
Cathepsin C limits acute viral infection independently of NK cell and CD8+ T‐cell cytolytic function
Immunology & Cell Biology (2011)
-
Cross-priming in health and disease
Nature Reviews Immunology (2010)