Abstract
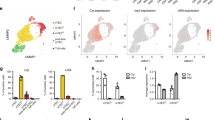
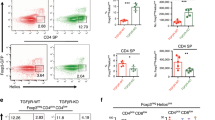
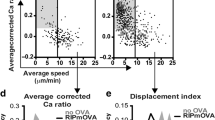
The parameters specifying whether autoreactive CD4+ thymocytes are deleted (recessive tolerance) or differentiate into regulatory T cells (dominant tolerance) remain unresolved. Dendritic cells directly delete thymocytes, partly through cross-presentation of peripheral antigens 'promiscuously' expressed in medullary thymic epithelial cells (mTECs) positive for the autoimmune regulator Aire. It is unclear if and how mTECs themselves act as antigen-presenting cells during tolerance induction. Here we found that an absence of major histocompatibility class II molecules on mTECs resulted in fewer polyclonal regulatory T cells. Furthermore, targeting of a model antigen to Aire+ mTECs led to the generation of specific regulatory T cells independently of antigen transfer to dendritic cells. Thus, 'routing' of mTEC-derived self antigens may determine whether specific thymocytes are deleted or enter the regulatory T cell lineage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Le Douarin, N. et al. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol. Rev. 149, 35–53 (1996).
Modigliani, Y. et al. Establishment of tissue-specific tolerance is driven by regulatory T cells selected by thymic epithelium. Eur. J. Immunol. 26, 1807–1815 (1996).
Shevach, E.M. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2, 389–400 (2002).
Maloy, K.J. & Powrie, F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2, 816–822 (2001).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Papiernik, M., de Moraes, M.L., Pontoux, C., Vasseur, F. & Penit, C. Regulatory CD4 T cells: expression of IL-2Rα chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10, 371–378 (1998).
Itoh, M. et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162, 5317–5326 (1999).
Hsieh, C.S., Zheng, Y., Liang, Y., Fontenot, J.D. & Rudensky, A.Y. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7, 401–410 (2006).
Pacholczyk, R., Ignatowicz, H., Kraj, P. & Ignatowicz, L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 25, 249–259 (2006).
Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3, 756–763 (2002).
Cabarrocas, J. et al. Foxp3+CD25+ regulatory T cells specific for a neo-self-antigen develop at the double-positive thymic stage. Proc. Natl. Acad. Sci. USA 103, 8453–8458 (2006).
Jordan, M.S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001).
Kawahata, K. et al. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol. 168, 4399–4405 (2002).
Klein, L., Khazaie, K. & von Boehmer, H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA 100, 8886–8891 (2003).
Lerman, M.A., Larkin, J., III, Cozzo, C., Jordan, M.S. & Caton, A.J. CD4+CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J. Immunol. 173, 236–244 (2004).
Walker, L.S., Chodos, A., Eggena, M., Dooms, H. & Abbas, A.K. Antigen-dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J. Exp. Med. 198, 249–258 (2003).
Bensinger, S.J., Bandeira, A., Jordan, M.S., Caton, A.J. & Laufer, T.M. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 194, 427–438 (2001).
Fontenot, J.D., Dooley, J.L., Farr, A.G. & Rudensky, A.Y. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202, 901–906 (2005).
Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2, 1032–1039 (2001).
Anderson, M.S. et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 298, 1395–1401 (2002).
Su, M.A. & Anderson, M.S. Aire: an update. Curr. Opin. Immunol. 16, 746–752 (2004).
Liston, A., Lesage, S., Wilson, J., Peltonen, L. & Goodnow, C.C. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4, 350–354 (2003).
Anderson, M.S. et al. The cellular mechanism of Aire control of T cell tolerance. Immunity 23, 227–239 (2005).
Hogquist, K.A., Baldwin, T.A. & Jameson, S.C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782 (2005).
Mathis, D. & Benoist, C. Back to central tolerance. Immunity 20, 509–516 (2004).
Kyewski, B. & Klein, L. A central role for central tolerance. Annu. Rev. Immunol. 24, 571–606 (2006).
Viret, C., Barlow, A.K. & Janeway, C.A., Jr. On the intrathymic intercellular transfer of self-determinants. Immunol. Today 20, 8–10 (1999).
Humblet, C., Rudensky, A. & Kyewski, B. Presentation and intercellular transfer of self antigen within the thymic microenvironment: expression of the Eα peptide-I-Ab complex by isolated thymic stromal cells. Int. Immunol. 6, 1949–1958 (1994).
Gallegos, A.M. & Bevan, M.J. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 200, 1039–1049 (2004).
Watanabe, N. et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436, 1181–1185 (2005).
Liu, Y.J. A unified theory of central tolerance in the thymus. Trends Immunol. 27, 215–221 (2006).
Kretschmer, K. et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6, 1219–1227 (2005).
Thorstenson, K.M. & Khoruts, A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167, 188–195 (2001).
Jenkinson, E.J. & Anderson, G. Fetal thymic organ cultures. Curr. Opin. Immunol. 6, 293–297 (1994).
Rodewald, H.R., Paul, S., Haller, C., Bluethmann, H. & Blum, C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature 414, 763–768 (2001).
van Meerwijk, J.P. et al. Quantitative impact of thymic clonal deletion on the T cell repertoire. J. Exp. Med. 185, 377–383 (1997).
Derbinski, J. et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202, 33–45 (2005).
Kirberg, J. et al. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 180, 25–34 (1994).
Brocker, T., Riedinger, M. & Karjalainen, K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185, 541–550 (1997).
Bot, A., Bot, S., Antohi, S., Karjalainen, K. & Bona, C. Kinetics of generation and persistence on membrane class II molecules of a viral peptide expressed on foreign and self proteins. J. Immunol. 157, 3436–3442 (1996).
Ribot, J., Romagnoli, P. & van Meerwijk, J.P. Agonist ligands expressed by thymic epithelium enhance positive selection of regulatory T lymphocytes from precursors with a normally diverse TCR repertoire. J. Immunol. 177, 1101–1107 (2006).
Rossi, S.W., Jenkinson, W.E., Anderson, G. & Jenkinson, E.J. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature 441, 988–991 (2006).
Bleul, C.C. et al. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature 441, 992–996 (2006).
Akiyama, T. et al. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science 308, 248–251 (2005).
King, C.G. et al. TRAF6 is a T cell-intrinsic negative regulator required for the maintenance of immune homeostasis. Nat. Med. 12, 1088–1092 (2006).
Klein, L., Roettinger, B. & Kyewski, B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur. J. Immunol. 31, 2476–2486 (2001).
Lohr, J., Knoechel, B., Kahn, E.C. & Abbas, A.K. Role of B7 in T cell tolerance. J. Immunol. 173, 5028–5035 (2004).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12, 431–440 (2000).
Tai, X., Cowan, M., Feigenbaum, L. & Singer, A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 6, 152–162 (2005).
Kishimoto, H. & Sprent, J. Several different cell surface molecules control negative selection of medullary thymocytes. J. Exp. Med. 190, 65–73 (1999).
van Santen, H.M., Benoist, C. & Mathis, D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J. Exp. Med. 200, 1221–1230 (2004).
Jiang, Q., Su, H., Knudsen, G., Helms, W. & Su, L. Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol. 7, 6 (2006).
Acknowledgements
We thank M.S. Anderson, B. Kyewski and J. Derbinski for comments on the manuscript. K. Karjalainen (Nanyung Technological University) provided the A5 hybridoma; A. Rudensky (University of Washington) provided Foxp3-specific polyclonal rabbit serum; R. Boyd (Monash Medical School) provided MTS10 supernatant; and B. Kyewski (German Cancer Research Center) provided biotinylated antibody to MHC class II. Supported by Boehringer Ingelheim (Research Institute of Molecular Pathology), the European Union (Euro-Thymaide FP6 Integrated Project; LSHB-CT-2003-503410) and the Austrian National Science Fund (Z58-B01 and Sonderforschungsbereich F023).
Author information
Authors and Affiliations
Contributions
K.A. generated and analyzed AIRE-HA × TCR-HA mice; L.M.D. did the RTOC experiments; E.H.V. generated and analyzed AIRE-OVA × DO11.10 mice together with J.E.; M.H. contributed to the generation and analysis of nude chimeras; L.K.S. and A.R. generated and analyzed CD11c-HA × TCR-HA mice (Supplementary Fig. 3); and L.K. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Generation of mixed RTOC, outline of the experimental procedure. (PDF 594 kb)
Supplementary Fig. 2
A distinct population of TCR-HA+CD25+Foxp3+ cells among peripheral CD4+ T cells in AIRE-HA × TCR-HA mice. (PDF 963 kb)
Supplementary Fig. 3
Targeting of HA to DCs in CD11c-HA × TCR-HA mice results in efficient intra-thymic deletion of TCR-HA+ cells. (PDF 76 kb)
Supplementary Fig. 4
Targeting of OVA to mTECs leads to generation of OVA–specific CD25+Foxp3+ Treg cells. (PDF 107 kb)
Supplementary Fig. 5
Presentation of HA(107-119) by DCs resulting from capture of mTEC-derived antigen. (PDF 57 kb)
Rights and permissions
About this article
Cite this article
Aschenbrenner, K., D'Cruz, L., Vollmann, E. et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8, 351–358 (2007). https://doi.org/10.1038/ni1444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1444
This article is cited by
-
The emerging family of RORγt+ antigen-presenting cells
Nature Reviews Immunology (2024)
-
B cells orchestrate tolerance to the neuromyelitis optica autoantigen AQP4
Nature (2024)
-
Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity
Cellular & Molecular Immunology (2023)
-
Embryonic keratin19+ progenitors generate multiple functionally distinct progeny to maintain epithelial diversity in the adult thymus medulla
Nature Communications (2023)
-
Autoimmune amelogenesis imperfecta in patients with APS-1 and coeliac disease
Nature (2023)