Abstract
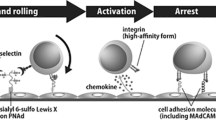
Lymphocyte homing is mediated by specific interaction between L-selectin on lymphocytes and the carbohydrate ligand 6-sulfo sialyl Lewis X on high endothelial venules. Here we generated mice lacking both core 1 extension and core 2 branching enzymes to assess the functions of O-glycan-borne L-selectin ligands in vivo. Mutant mice maintained robust lymphocyte homing, yet they lacked O-glycan L-selectin ligands. Biochemical analyses identified a class of N-glycans bearing the 6-sulfo sialyl Lewis X L-selectin ligand in high endothelial venules. These N-glycans supported the binding of L-selectin to high endothelial venules in vitro and contributed in vivo to O-glycan-independent lymphocyte homing in wild-type and mutant mice. Our results demonstrate the critical function of N-glycan-linked 6-sulfo sialyl Lewis X in L-selectin-dependent lymphocyte homing and recruitment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Marchesi, V.T. & Gowans, J.L. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc. R. Soc. Lond. B Biol. Sci. 159, 283–290 (1964).
Arbones, M.L. et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1, 247–260 (1994).
Rosen, S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 (2004).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Martin-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat. Immunol. 5, 1260–1265 (2004).
Chen, S., Kawashima, H., Lowe, J.B., Lanier, L.L. & Fukuda, M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J. Exp. Med. 202, 1679–1689 (2005).
Maly, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Homeister, J.W. et al. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15, 115–126 (2001).
Kawashima, H. et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 6, 1096–1104 (2005).
Uchimura, K. et al. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat. Immunol. 6, 1105–1113 (2005).
Hiraoka, N. et al. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl LewisX, an L-selectin ligand displayed by CD34. Immunity 11, 79–89 (1999).
Bierhuizen, M.F. & Fukuda, M. Expression cloning of a cDNA encoding UDP-GlcNAc:Galβ1-3-GalNAc-R (GlcNAc to GalNAc)β1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc. Natl. Acad. Sci. USA 89, 9326–9330 (1992).
Ellies, L.G. et al. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity 9, 881–890 (1998).
Hiraoka, N. et al. Core 2 branching β1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J. Biol. Chem. 279, 3058–3067 (2004).
Yeh, J.C. et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3-N-acetylglucosaminyltransferase. Cell 105, 957–969 (2001).
Streeter, P.R., Rouse, B.T. & Butcher, E.C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J. Cell Biol. 107, 1853–1862 (1988).
Aoki, D., Lee, N., Yamaguchi, N., Dubois, C. & Fukuda, M.N. Golgi retention of a trans-Golgi membrane protein, galactosyltransferase, requires cysteine and histidine residues within the membrane-anchoring domain. Proc. Natl. Acad. Sci. USA 89, 4319–4323 (1992).
M'Rini, C. et al. A novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by α(1,3)-fucosyltransferase-IV. J. Exp. Med. 198, 1301–1312 (2003).
Gauguet, J.M., Rosen, S.D., Marth, J.D. & von Andrian, U.H. Core 2 branching β1,6-N-acetylglucosaminyltransferase and high endothelial cell N-acetylglucosamine-6-sulfotransferase exert differential control over B- and T-lymphocyte homing to peripheral lymph nodes. Blood 104, 4104–4112 (2004).
Hemmerich, S., Leffler, H. & Rosen, S.D. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J. Biol. Chem. 270, 12035–12047 (1995).
Kanamori, A. et al. Distinct sulfation requirements of selectins disclosed using cells that support rolling mediated by all three selectins under shear flow. L-selectin prefers carbohydrate 6-sulfation totyrosine sulfation, whereas P-selectin does not. J. Biol. Chem. 277, 32578–32586 (2002).
Lasky, L.A. et al. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell 69, 927–938 (1992).
Wang, L., Fuster, M., Sriramarao, P. & Esko, J.D. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 6, 902–910 (2005).
Lowe, J.B. Glycosylation, immunity, and autoimmunity. Cell 104, 809–812 (2001).
Laakkonen, P., Porkka, K., Hoffman, J.A. & Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 8, 751–755 (2002).
Cummings, R.D. & Kornfield, S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J. Biol. Chem. 257, 11230–11234 (1982).
Rosen, S.D., Tsay, D., Singer, M.S., Hemmerich, S. & Abraham, W.M. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am. J. Pathol. 166, 935–944 (2005).
Finger, E.B. et al. Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 379, 266–269 (1996).
Steegmaier, M. et al. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature 373, 615–620 (1995).
Clark, R.A., Fuhlbrigge, R.C. & Springer, T.A. L-selectin ligands that are O-glycoprotease resistant and distinct from MECA-79 antigen are sufficient for tethering and rolling of lymphocytes on human high endothelial venules. J. Cell. Biol. 140, 721–731 (1998).
Liu, W. et al. Identification of N-terminal residues on P-selectin glycoprotein ligand-1 required for binding to P-selectin. J. Biol. Chem. 273, 7078–7087 (1998).
Leppanen, A., Yago, T., Otto, V.I., McEver, R.P. & Cummings, R.D. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J. Biol. Chem. 278, 26391–26400 (2003).
Martinez, M. et al. Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII. J. Biol. Chem. 280, 5378–5390 (2005).
Baumheter, S. et al. Binding of L-selectin to the vascular sialomucin CD34. Science 262, 436–438 (1993).
Sassetti, C., Tangemann, K., Singer, M.S., Kershaw, D.B. & Rosen, S.D. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J. Exp. Med. 187, 1965–1975 (1998).
Morgan, S.M., Samulowitz, U., Darley, L., Simmons, D.L. & Vestweber, D. Biochemical characterization and molecular cloning of a novel endothelial-specific sialomucin. Blood 93, 165–175 (1999).
Fieger, C.B., Sassetti, C.M. & Rosen, S.D. Endoglycan, a member of the CD34 family, functions as an L-selectin ligand through modification with tyrosine sulfation and sialyl Lewis x. J. Biol. Chem. 278, 27390–27398 (2003).
Briskin, M.J., McEvoy, L.M. & Butcher, E.C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature 363, 461–464 (1993).
Coltart, D.M. et al. Principles of mucin architecture: structural studies on synthetic glycopeptides bearing clustered mono-, di-, tri-, and hexasaccharide glycodomains. J. Am. Chem. Soc. 124, 9833–9844 (2002).
Cyster, J.G., Shotton, D.M. & Williams, A.F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 10, 893–902 (1991).
Li, F. et al. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J. Biol. Chem. 271, 6342–6348 (1996).
Phan, U.T., Waldron, T.T. & Springer, T.A. Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat. Immunol. 7, 883–889 (2006).
Shimaoka, M. et al. Structures of the αL I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99–111 (2003).
Shamri, R. et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 6, 497–506 (2005).
Ley, K., Tedder, T.F. & Kansas, G.S. L-selectin can mediate leukocyte rolling in untreated mesenteric venules in vivo independent of E- or P-selectin. Blood 82, 1632–1638 (1993).
Ohmori, K. et al. Identification of cutaneous lymphocyte-associated antigen as sialyl 6-sulfo Lewis X, a selectin ligand expressed on a subset of skin-homing helper memory T cells. Blood 107, 3197–3204 (2006).
Kobayashi, M. et al. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc. Natl. Acad. Sci. USA 101, 17807–17812 (2004).
Renkonen, J., Tynninen, O., Hayry, P., Paavonen, T. & Renkonen, R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am. J. Pathol. 161, 543–550 (2002).
van Zante, A. et al. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. J. Exp. Med. 198, 1289–1300 (2003).
Mitoma, J. et al. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis X-type L-selectin ligand activity. J. Biol. Chem. 278, 9953–9961 (2003).
Mitoma, J. & Fukuda, M. in Methods in Enzymology vol. 416 (ed. Fukuda, M.) 293–304 (Academic, San Diego, 2006).
Acknowledgements
We thank S. Rosen, R. Cummings and M.N. Fukuda for discussions; Y. Altman for cell sorting; K. Sun for technical assistance; E. Lamar for critical reading of the manuscript; and A. Morse and T. Mabry for organizing the manuscript. S. Rosen (University of California at San Francisco) provided 38C13 B lymphoma cells. Supported by the National Institutes of Health (CA48737 to M.F.; P01 CA71932 to M.F. and J.B.L.; AI061663, AI069259 and AR42689 to U.H.v.A.; and DK48247 to J.D.M.) and the Taiwan National Science Council (95-3112-B-001-014 to the National Core Facilities for Proteomics (for mass spectrometry)).
Author information
Authors and Affiliations
Contributions
J.M. and X.B. did most of the experiments; B.P. did the rolling assays; P.S. and J.-M.G. did intravital microscopy; S.-Y.Y. did mass spectrometry; H.K. and H.S. assisted with the lymphocyte homing assay; K.O. and J.D.M. did embryonic stem cell culture; J.M., X.B., K.-H.K., U.H.v.A. and J.B.L. contributed to the preparation of the manuscript; and M.F. conceptualized the work, organized all experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Structure and biosynthesis of L-selectin ligands and Core1-β3GlcNAc knockout. (PDF 401 kb)
Supplementary Fig. 2
MECA-79 antibody inhibited lymphocyte homing in WT but not in Core1-β3GlcNAcT (C1-E) KO mice. (PDF 235 kb)
Supplementary Fig. 3
Effects of enzymatic digestions on binding of L-selectin-IgM to HEV. (PDF 162 kb)
Supplementary Fig. 4
Binding of N-glycan-specific lectins in HEV and their effects in inhibiting L-selectin-IgM binding. (PDF 439 kb)
Supplementary Fig. 5
MALDI-MS analysis of permethylated, sulfated N-glycans in negative ion mode. (PDF 663 kb)
Supplementary Fig. 6
Specificity of anti-CD34 antibody and number of leukocytes and CD3+ lymphocytes in contact hypersensitivity (CHS). (PDF 353 kb)
Supplementary Table 1
Tentative assignment of the MALDI-MS molecular ion signals afforded by the permethylated sulfated N-glycans in negative ion mode. (PDF 74 kb)
Rights and permissions
About this article
Cite this article
Mitoma, J., Bao, X., Petryanik, B. et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol 8, 409–418 (2007). https://doi.org/10.1038/ni1442
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1442
This article is cited by
-
CHST2-mediated sulfation of MECA79 antigens is critical for breast cancer cell migration and metastasis
Cell Death & Disease (2023)
-
Immunomodulation by endothelial cells — partnering up with the immune system?
Nature Reviews Immunology (2022)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
HIV-1 targets L-selectin for adhesion and induces its shedding for viral release
Nature Communications (2018)
-
Protein N-Glycosylation in Cardiovascular Diseases and Related Risk Factors
Current Cardiovascular Risk Reports (2018)