Abstract
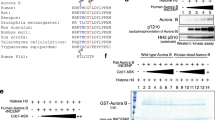
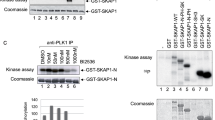
CD28-deficient T cells arrest at the G1–S transition of the cell cycle. Here we show that this is controlled by the kinase aurora B, which exists in a complex with survivin and mammalian target of rapamycin (mTOR). Expression of aurora B in Cd28−/− T cells augmented phosphorylation of mTOR substrates, expression of cyclin A, hyperphosphorylation of retinoblastoma protein and activation of cyclin-dependent kinases 1 and 2 and promoted cell cycle progression. Interleukin 2 enhanced aurora B activity, and inactive aurora B prevented interleukin 2–induced proliferation. Moreover, expression of aurora B restored Cd28−/− T cell proliferation and promoted inflammation in vivo. These data identify aurora B, along with survivin and mTOR, as a regulator of the G1–S checkpoint in T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Lafferty, K.J. & Cunningham, A.J. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci. 53, 27–42 (1975).
Jenkins, M.K. & Schwartz, R.H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165, 302–319 (1987).
June, C.H., Ledbetter, J.A., Gillespie, M.M., Lindsten, T. & Thompson, C.B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol. Cell. Biol. 7, 4472–4481 (1987).
Linsley, P.S. et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 173, 721–730 (1991).
Harding, F.A., McArthur, J.G., Gross, J.A., Raulet, D.H. & Allison, J.P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356, 607–609 (1992).
Sharpe, A.H. & Freeman, G.J. The B7–CD28 superfamily. Nat. Rev. Immunol. 2, 116–126 (2002).
Croft, M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3, 609–620 (2003).
Beverly, B., Kang, S.M., Lenardo, M.J. & Schwartz, R.H. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 4, 661–671 (1992).
Boussiotis, V.A. et al. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science 266, 1039–1042 (1994).
Powell, J.D., Lerner, C.G. & Schwartz, R.H. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J. Immunol. 162, 2775–2784 (1999).
Prasad, K.V. et al. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc. Natl. Acad. Sci. USA 91, 2834–2838 (1994).
Ahmed, N.N., Grimes, H.L., Bellacosa, A., Chan, T.O. & Tsichlis, P.N. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc. Natl. Acad. Sci. USA 94, 3627–3632 (1997).
Nourse, J. et al. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372, 570–573 (1994).
Boussiotis, V.A. et al. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat. Med. 6, 290–297 (2000).
Powell, J.D., Bruniquel, D. & Schwartz, R.H. TCR engagement in the absence of cell cycle progression leads to T cell anergy independent of p27Kip1. Eur. J. Immunol. 31, 3737–3746 (2001).
Kane, L.P., Andres, P.G., Howland, K.C., Abbas, A.K. & Weiss, A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-γ but not TH2 cytokines. Nat. Immunol. 2, 37–44 (2001).
Song, J., So, T., Cheng, M., Tang, X. & Croft, M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity 22, 621–631 (2005).
Li, F. et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396, 580–584 (1998).
Tamm, I. et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58, 5315–5320 (1998).
Li, F. et al. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat. Cell Biol. 1, 461–466 (1999).
Wheatley, S.P., Carvalho, A., Vagnarelli, P. & Earnshaw, W.C. INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr. Biol. 11, 886–890 (2001).
Bolton, M.A. et al. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell 13, 3064–3077 (2002).
Terada, Y. et al. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 17, 667–676 (1998).
Murata-Hori, M. & Wang, Y.L. The kinase activity of aurora B is required for kinetochore-microtubule interactions during mitosis. Curr. Biol. 12, 894–899 (2002).
Kallio, M.J., McCleland, M.L., Stukenberg, P.T. & Gorbsky, G.J. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 12, 900–905 (2002).
Hunter, T. & Pines, J. Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell 79, 573–582 (1994).
Ekholm, S.V. & Reed, S.I. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12, 676–684 (2000).
Yam, C.H., Fung, T.K. & Poon, R.Y. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 59, 1317–1326 (2002).
Morice, W.G., Wiederrecht, G., Brunn, G.J., Siekierka, J.J. & Abraham, R.T. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J. Biol. Chem. 268, 22737–22745 (1993).
Murata-Hori, M., Tatsuka, M. & Wang, Y.L. Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol. Biol. Cell 13, 1099–1108 (2002).
O'Connor, D.S. et al. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc. Natl. Acad. Sci. USA 97, 13103–13107 (2000).
Wall, N.R., O'Connor, D.S., Plescia, J., Pommier, Y. & Altieri, D.C. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 63, 230–235 (2003).
Wheatley, S.P., Henzing, A.J., Dodson, H., Khaled, W. & Earnshaw, W.C. Aurora-B phosphorylation in vitro identifies a residue of survivin that is essential for its localization and binding to inner centromere protein (INCENP) in vivo. J. Biol. Chem. 279, 5655–5660 (2004).
Chen, J. et al. Survivin enhances Aurora-B kinase activity and localizes Aurora-B in human cells. J. Biol. Chem. 278, 486–490 (2003).
Morice, W.G., Brunn, G.J., Wiederrecht, G., Siekierka, J.J. & Abraham, R.T. Rapamycin-induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J. Biol. Chem. 268, 3734–3738 (1993).
Inoki, K., Ouyang, H., Li, Y. & Guan, K.L. Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol. Biol. Rev. 69, 79–100 (2005).
Martin, D.E. & Hall, M.N. The expanding TOR signaling network. Curr. Opin. Cell Biol. 17, 158–166 (2005).
Salek-Ardakani, S. et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 198, 315–324 (2003).
Carmena, M. & Earnshaw, W.C. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 4, 842–854 (2003).
Ke, Y.W., Dou, Z., Zhang, J. & Yao, X.B. Function and regulation of Aurora/Ipl1p kinase family in cell division. Cell Res. 13, 69–81 (2003).
Xing, Z., Conway, E.M., Kang, C. & Winoto, A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J. Exp. Med. 199, 69–80 (2004).
Okada, H. et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J. Exp. Med. 199, 399–410 (2004).
Suzuki, A. et al. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16INK4a and Cdk2/cyclin E complex activation. Oncogene 19, 3225–3234 (2000).
Fukuda, S. & Pelus, L.M. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34+ cells by hematopoietic growth factors: implication of survivin expression in normal hematopoiesis. Blood 98, 2091–2100 (2001).
Shen, W.H. et al. Tumor necrosis factor alpha inhibits cyclin A expression and retinoblastoma hyperphosphorylation triggered by insulin-like growth factor-I induction of new E2F–1 synthesis. J. Biol. Chem. 279, 7438–7446 (2004).
Suzuki, A. et al. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene 19, 1346–1353 (2000).
Jacinto, E. & Hall, M.N. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell Biol. 4, 117–126 (2003).
Chiang, G.G. & Abraham, R.T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 280, 25485–25490 (2005).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 (2002).
Acknowledgements
We thank M. Tatsuka (Research Institute for Radiation Biology and Medicine, Hiroshima University) for aurora B cDNA, and A. Song, W. Duan, X. Tang and Y. Adams for technical assistance. The cDNA to construct Mig vectors expressing survivin and dominant negative survivin was provided by D. Altieri (University of Massachusetts Medical School). Supported by the US National Institutes of Health (AI50498 and AI49453 to M.C.) and the Universitywide AIDS Research Program (J.S.). This is manuscript 764 from the La Jolla Institute for Allergy and Immunology.
Author information
Authors and Affiliations
Contributions
J.S., S.S.-A., T.S. and M.C. designed the research and analyzed the data; J.S., S.S.-A. and T.S. did the research; and M.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Aurora B is expressed and active in T cells. (PDF 55 kb)
Supplementary Fig. 2
Aurora B expression and activity in naive T cells. (PDF 71 kb)
Supplementary Fig. 3
MEK/Erk regulate aurora B activity and G1-S transition. (PDF 264 kb)
Supplementary Fig. 4
Aurora B activity induced by IL-2R is dependent on mTor. (PDF 70 kb)
Rights and permissions
About this article
Cite this article
Song, J., Salek-Ardakani, S., So, T. et al. The kinases aurora B and mTOR regulate the G1–S cell cycle progression of T lymphocytes. Nat Immunol 8, 64–73 (2007). https://doi.org/10.1038/ni1413
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1413
This article is cited by
-
Effect of rapamycin treatment in human seminoma TCam-2 cells through inhibition of G1-S transition
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Nanoparticle-Mediated Lipid Metabolic Reprogramming of T Cells in Tumor Microenvironments for Immunometabolic Therapy
Nano-Micro Letters (2021)
-
Toxoplasma gondii chromosomal passenger complex is essential for the organization of a functional mitotic spindle: a prerequisite for productive endodyogeny
Cellular and Molecular Life Sciences (2018)
-
Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression
Nature Immunology (2017)
-
Ku70 Serine 155 mediates Aurora B inhibition and activation of the DNA damage response
Scientific Reports (2016)