Abstract
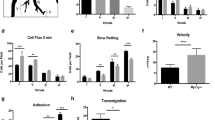
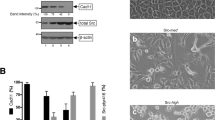
The migration of B cells into secondary lymphoid organs is required for the generation of an effective immune response. Here we analyzed the involvement of SWAP-70, a Rac-interacting protein involved in actin rearrangement, in B cell entry into lymph nodes. We noted reduced migration of Swap70−/− B cells into lymph nodes in vivo. Swap70−/− B cells rolled and adhered, yet accumulated in lymph node high endothelial venules. This defect was not due to impaired integrin expression or chemotaxis. Instead, Swap70−/− B cells aberrantly regulated integrin-mediated adhesion. During attachment, Swap70−/− B cells showed defective polarization and did not form uropods or stabilize lamellipodia at a defined region. Thus, SWAP-70 selectively regulates processes essential for B cell entry into lymph nodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Campbell, J.J. & Butcher, E.C. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12, 336–341 (2000).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Warnock, R.A., Askari, S., Butcher, E.C. & von Andrian, U.H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 187, 205–216 (1998).
Ngo, V.N., Cornall, R.J. & Cyster, J.G. Splenic T zone development is B cell dependent. J. Exp. Med. 194, 1649–1660 (2001).
Nolte, M.A. et al. B cells are crucial for both development and maintenance of the splenic marginal zone. J. Immunol. 172, 3620–3627 (2004).
Angeli, V. et al. B cell driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 (2006).
Wittchen, E.S., van Buul, J.D., Burridge, K. & Worthylake, R.A. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Curr. Opin. Hematol. 12, 14–21 (2005).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Fenteany, G. & Glogauer, M. Cytoskeletal remodeling in leukocyte function. Curr. Opin. Hematol. 11, 15–24 (2004).
Sun, C.X. et al. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104, 3758–3765 (2004).
Weiss-Haljiti, C. et al. Involvement of phosphoinositide 3-kinase-γ, Rac, and PAK signaling in chemokine-induced macrophage migration. J. Biol. Chem. 279, 43273–43284 (2004).
Filippi, M.D. et al. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 5, 744–751 (2004).
Gakidis, M.A. et al. Vav GEFs are required for β2 integrin-dependent functions of neutrophils. J. Cell Biol. 166, 273–282 (2004).
Gismondi, A. et al. Proline-rich tyrosine kinase 2 and Rac activation by chemokine and integrin receptors controls NK cell transendothelial migration. J. Immunol. 170, 3065–3073 (2003).
Curnock, A.P., Logan, M.K. & Ward, S.G. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology 105, 125–136 (2002).
Nombela-Arrieta, C. et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity 21, 429–441 (2004).
Borggrefe, T. et al. Cellular, intracellular, and developmental expression patterns of murine SWAP-70. Eur. J. Immunol. 29, 1812–1822 (1999).
Shinohara, M. et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature 416, 759–763 (2002).
Tanaka, Y. et al. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity 18, 403–414 (2003).
Gupta, S. et al. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum. Immunol. 64, 389–401 (2003).
Hilpela, P. et al. SWAP-70 identifies a transitional subset of actin filaments in motile cells. Mol. Biol. Cell 14, 3242–3253 (2003).
Ihara, S., Oka, T. & Fukui, Y. Direct binding of SWAP-70 to non-muscle actin is required for membrane ruffling. J. Cell Sci. 119, 500–507 (2006).
Sivalenka, R.R. & Jessberger, R. SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol. Cell. Biol. 24, 10277–10288 (2004).
Hogg, N., Laschinger, M., Giles, K. & McDowall, A. T-cell integrins: more than just sticking points. J. Cell Sci. 116, 4695–4705 (2003).
Dustin, M.L., Bivona, T.G. & Philips, M.R. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 5, 363–372 (2004).
Prasad, K.S. et al. Soluble CD40 ligand induces β3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc. Natl. Acad. Sci. USA 100, 12367–12371 (2003).
Davey, E.J. et al. Homotypic aggregation of murine B lymphocytes is independent of CD23. Eur. J. Immunol. 25, 1224–1229 (1995).
Greicius, G., Tamosiunas, V. & Severinson, E. Assessment of the role of leucocyte function-associated antigen-1 in homotypic adhesion of activated B lymphocytes. Scand. J. Immunol. 48, 642–650 (1998).
Borggrefe, T., Keshavarzi, S., Gross, B., Wabl, M. & Jessberger, R. Impaired IgE response in SWAP-70-deficient mice. Eur. J. Immunol. 31, 2467–2475 (2001).
Smith, A., Bracke, M., Leitinger, B., Porter, J.C. & Hogg, N. LFA-1-induced T cell migration on ICAM-1 involves regulation of MLCK-mediated attachment and ROCK-dependent detachment. J. Cell Sci. 116, 3123–3133 (2003).
Davey, E.J., Thyberg, J., Conrad, D.H. & Severinson, E. Regulation of cell morphology in B lymphocytes by IL-4: evidence for induced cytoskeletal changes. J. Immunol. 160, 5366–5373 (1998).
Smith, A. et al. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J. Cell Biol. 170, 141–151 (2005).
Campbell, J.J. et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279, 381–384 (1998).
McLachlan, J.B. et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 4, 1199–1205 (2003).
Wang, F. et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4, 513–518 (2002).
Xu, J. et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214 (2003).
Worthylake, R.A. & Burridge, K. RhoA and ROCK promote migration by limiting membrane protrusions. J. Biol. Chem. 278, 13578–13584 (2003).
Schenkel, A.R., Mamdouh, Z. & Muller, W.A. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5, 393–400 (2004).
Dustin, M.L., Carpen, O. & Springer, T.A. Regulation of locomotion and cell-cell contact area by the LFA-1 and ICAM-1 adhesion receptors. J. Immunol. 148, 2654–2663 (1992).
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2, 515–522 (2001).
Millan, J. et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 8, 113–123 (2006).
Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
von Andrian, U.H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996).
von Andrian, U.H. & M'Rini, C. In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes. Commun. 6, 85–96 (1998).
Masat, L. et al. Association of SWAP-70 with the B cell antigen receptor complex. Proc. Natl. Acad. Sci. USA 97, 2180–2184 (2000).
Acknowledgements
We thank L. Quemeneur for discussions, and A. Berg and M. Chopin for help. Supported by the National Institutes of Health (AI49470 to R.J.) and the Deutsche Forschungsgemeinschaft (SFB605 to R.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Inflammation-induced migration of B cells into the peritoneum. (PDF 207 kb)
Supplementary Fig. 2
Flow cytometric analysis of integrin and chemokine receptor expression on purified B cells. (PDF 692 kb)
Supplementary Fig. 3
Actin polymerization and Rac-1 activity and localization. (PDF 531 kb)
Supplementary Video 1
WT B cells moving on an anti-CD44 coated surface. Images were collected every 10 sec for 20 min after a 25 min attachment period. (MOV 3624 kb)
Supplementary Video 2
Swap70−/− B cells moving on an anti-CD44 coated surface. Images were collected every 10 sec for 20 min after a 25 min attachment period. (MOV 3100 kb)
Rights and permissions
About this article
Cite this article
Pearce, G., Angeli, V., Randolph, G. et al. Signaling protein SWAP-70 is required for efficient B cell homing to lymphoid organs. Nat Immunol 7, 827–834 (2006). https://doi.org/10.1038/ni1365
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1365
This article is cited by
-
SWAP-70 promotes glioblastoma cellular migration and invasion by regulating the expression of CD44s
Cancer Cell International (2019)
-
At the intersection of DNA damage and immune responses
Nature Reviews Immunology (2019)
-
Transient Cannabinoid Receptor 2 Blockade during Immunization Heightens Intensity and Breadth of Antigen-specific Antibody Responses in Young and Aged mice
Scientific Reports (2017)
-
The guanine exchange factor SWAP70 mediates vGPCR-induced endothelial plasticity
Cell Communication and Signaling (2015)
-
Stage-specific control of early B cell development by the transcription factor Ikaros
Nature Immunology (2014)